
Back ۲٬۳-بوتاندیول AZB 2,3-Butandiol German 2,3-Butanoduolo Esperanto 2,3-butanodiol Spanish ۳٬۲-بوتاندیول Persian 2,3-butaanidioli Finnish Butane-2,3-diol French 2,3-ブタンジオール Japanese 2,3-뷰테인다이올 Korean Butano-2,3-diol Portuguese
| |
| Names | |
|---|---|
| Preferred IUPAC name
Butane-2,3-diol | |
| Other names
2,3-Butylene glycol
Pseudobutylene glycol 2,3-Dihydroxybutane Butan-2,3-diol Diethanol[citation needed] & Bis-ethanol | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.431 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H10O2 | |
| Molar mass | 90.122 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | odorless |
| Density | 0.987 g/mL |
| Melting point | 19 °C (66 °F; 292 K) |
| Boiling point | 177 °C (351 °F; 450 K) |
| Miscible | |
| Solubility in other solvents | Soluble in alcohol, ketones, ether |
| log P | -0.92 |
| Vapor pressure | 0.23 hPa (20 °C) |
| Acidity (pKa) | 14.9 |
Refractive index (nD)
|
1.4366 |
| Thermochemistry | |
Heat capacity (C)
|
213.0 J/K mol |
Std enthalpy of
formation (ΔfH⦵298) |
-544.8 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 85 °C (185 °F; 358 K) |
| 402 °C (756 °F; 675 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
5462 mg/kg (rat, oral) |
| Related compounds | |
Related butanediols
|
1,4-Butanediol 1,3-Butanediol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
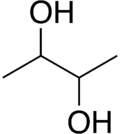
2,3-Butanediol is the organic compound with the formula (CH3CHOH)2. It is classified as a vic-diol (glycol). It exists as three stereoisomers, a chiral pair and the meso isomer. All are colorless liquids. Applications include precursors to various plastics and pesticides.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
