
Back بيفلوريد الأمونيوم Arabic آمونیوم بیفلوئورید AZB Hydrogendifluorid amonný Czech Ammoniumhydrogendifluorid German Amonia bifluorido Esperanto Bifluoruro de amonio Spanish آمونیوم بیفلوئورید Persian Ammoniumvetyfluoridi Finnish Bifluorure d'ammonium French Amonium bifluorida ID
| |||

| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ammonium bifluoride
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.014.252 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 1727 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| [NH4][HF2] | |||
| Molar mass | 57.044 g·mol−1 | ||
| Appearance | Colourless crystals | ||
| Density | 1.50 g cm−3 | ||
| Melting point | 126 °C (259 °F; 399 K) | ||
| Boiling point | 240 °C (464 °F; 513 K)(decomposes) | ||
| 63g/(100 ml) (20 °C) | |||
| Solubility in alcohol | slightly soluble | ||
Refractive index (nD)
|
1.390 | ||
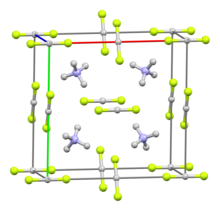
| Structure | |||
| Cubic, related to the CsCl structure | |||
| [NH4]+ cation: tetrahedral [HF2]− anion: linear | |||
| Hazards | |||
| GHS labelling: | |||
  [1] [1]
| |||
| H301, H314[1] | |||
| P280, P301+P310, P305+P351+P338, P310[1] | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Other cations
|
potassium bifluoride | ||
Related compounds
|
ammonium fluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ammonium bifluoride is the inorganic compound with the formula [NH4][HF2] or [NH4]F·HF. It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-etchant and an intermediate in a once-contemplated route to hydrofluoric acid.
- ^ a b c Sigma-Aldrich Co., Ammonium bifluoride. Retrieved on 2013-07-20.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search


