
Back نتريت أمونيوم Arabic نیتریت آمونیوم AZB Nitrit d'amoni Catalan Dusitan amonný Czech Ammoniumnitrit German Amonia nitrito Esperanto Nitrito de amonio Spanish نیتریت آمونیوم Persian Ammoniumnitriitti Finnish Amonium nitrit ID
| |
| |
| Names | |
|---|---|
| IUPAC name
Ammonium nitrite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.257 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
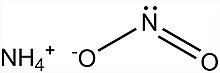
| [NH4]NO2 | |
| Molar mass | 64.044 g·mol−1 |
| Appearance | colorless or pale yellow crystals |
| Density | 1.69 g/cm3 |
| Melting point | Decomposes |
| 118.3 g / 100mL | |
| Explosive data | |
| Shock sensitivity | Low |
| Friction sensitivity | Low |
| Detonation velocity | >1000 m/s |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Explosive |
| GHS labelling: | |
 
| |
| Danger | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Non-flammable | |
| Related compounds | |
Other anions
|
Ammonium nitrate |
Other cations
|
Sodium nitrite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
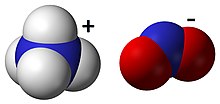
Ammonium nitrite is a chemical compound with the chemical formula [NH4]NO2. It is the ammonium salt of nitrous acid. It is composed of ammonium cations [NH4]+ and nitrite anions NO−2. It is not used in pure isolated form since it is highly unstable and decomposes into water and nitrogen, even at room temperature.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
