
Back نتريد الكالسيوم Arabic نیترید کلسیوم AZB Nitrur de calci Catalan Кальций нитри(д)чĕ CV Calciumnitrid German Nitruro de calcio Spanish کلسیم نیترید Persian Nitrure de calcium French Kalcium-nitrid Hungarian Nitruro di calcio Italian
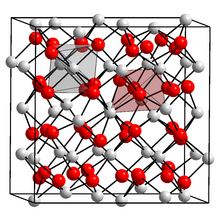 Unit cell containing 31 nitride ions (red) and 48 calcium ions (white). Each nitride is surrounded by six calcium, and each calcium by four nitride ions.
| |
| Names | |
|---|---|
| IUPAC name
Calcium nitride
| |
| Other names
tricalcium dinitride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.031.435 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Ca3N2 | |
| Molar mass | 148.248 g·mol−1 |
| Appearance | red-brown crystalline solid |
| Density | 2.670 g/cm3 2.63 g/cm3 (17 °C) |
| Melting point | 1,195 °C (2,183 °F; 1,468 K) |
| decomposes | |
| Structure | |
| Cubic, cI80 | |
| Ia-3, No. 206 | |
| Related compounds | |
Other anions
|
|
Other cations
|
Beryllium nitride Magnesium nitride Strontium nitride Barium nitride Radium nitride Zinc nitride Aluminium nitride Lithium nitride Sodium nitride Potassium nitride |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Calcium nitride is the inorganic compound with the chemical formula Ca3N2.[1] It exists in various forms (isomorphs), α-calcium nitride being more commonly encountered.
- ^ Eagleson, M. (1994). Concise Encyclopedia Chemistry. Walter de Gruyter. p. 160. ISBN 3-11-011451-8.
Calcium nitride.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search