
Back Cannabigerol German Cannabigerol Spanish Cannabigerol French Kannabigerol Hungarian Cannabigerolo Italian Kannabigerol Polish Каннабигерол Russian Kanabigerol Serbo-Croatian Kanabigerol Serbian
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.346.098 |
| Chemical and physical data | |
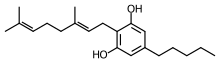
| Formula | C21H32O2 |
| Molar mass | 316.485 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |

Cannabigerol (CBG) is one of more than 120 identified cannabinoid compounds found in the plant genus Cannabis.[1][2] Cannabigerol is the decarboxylated form of cannabigerolic acid, the parent molecule from which other cannabinoids are synthesized.[3][4]
Cannabigerol is normally a minor constituent of cannabis.[3][5] During plant growth, most of the cannabigerol is converted into other cannabinoids, primarily tetrahydrocannabinol (THC) or cannabidiol (CBD), leaving about 1% cannabigerol in the plant.[6] Some strains, however, produce larger amounts of cannabigerol and cannabigerolic acid, while having low quantities of other cannabinoids, like THC and CBD.[7]
Although cannabigerol is sold as a dietary supplement, its effects and safety for human consumption are undefined.[3]
- ^ ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A (2017). "Phytochemistry of Cannabis sativa L". Phytochemistry of Cannabis sativa L. Progress in the Chemistry of Organic Natural Products. Vol. 103. pp. 1–36. doi:10.1007/978-3-319-45541-9_1. ISBN 978-3-319-45539-6. PMID 28120229.
- ^ Turner SE, Williams CM, Iversen L, Whalley BJ (2017). "Molecular Pharmacology of Phytocannabinoids". Phytocannabinoids. Progress in the Chemistry of Organic Natural Products. Vol. 103. pp. 61–101. doi:10.1007/978-3-319-45541-9_3. ISBN 978-3-319-45539-6. PMID 28120231.
- ^ a b c Nachnani R, Raup-Konsavage WM, Vrana KE (2021). "The pharmacological case for cannabigerol". The Journal of Pharmacology and Experimental Therapeutics. 376 (2): 204–212. doi:10.1124/jpet.120.000340. ISSN 0022-3565. PMID 33168643. S2CID 226296897.
- ^ "Cannabigerol; ID 5315659". PubChem, National Library of Medicine, US National Institutes of Health. 2 July 2022. Retrieved 7 July 2022.
- ^ Morales P, Reggio PH, Jagerovic N (2017). "An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol". Frontiers in Pharmacology. 8: 422. doi:10.3389/fphar.2017.00422. PMC 5487438. PMID 28701957.
- ^ Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. (February 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–331. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472.
- ^ Zagožen M, Čerenak A, Kreft S (2021-09-01). "Cannabigerol and cannabichromene in Cannabis sativa L." Acta Pharmaceutica. 71 (3): 355–364. doi:10.2478/acph-2021-0021. PMID 36654096. S2CID 231543630.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search