
Back كابسيسين Arabic کپسایسین AZB Капсаицин Bulgarian Kapsaicin BS Capsaïcina Catalan Kapsaicin Czech Capsaicin Welsh Capsaicin Danish Capsaicin German Καψαϊκίνη Greek
| |

| |
| Names | |
|---|---|
| Pronunciation | /kæpˈseɪsɪn/ or /kæpˈseɪəsɪn/ |
| Preferred IUPAC name
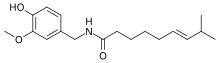
(6E)-N-[(4-Hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide | |
| Other names
(E)-N-(4-Hydroxy-3-methoxybenzyl)-8-methylnon-6-enamide
8-Methyl-N-vanillyl-trans-6-nonenamide trans-8-Methyl-N-vanillylnon-6-enamide (E)-Capsaicin Capsicine Capsicin CPS | |
| Identifiers | |
3D model (JSmol)
|
|
| 2816484 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.337 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H27NO3 | |
| Molar mass | 305.418 g·mol−1 |
| Appearance | Crystalline white powder[1] |
| Odor | Highly pungent |
| Melting point | 62 to 65 °C (144 to 149 °F; 335 to 338 K) |
| Boiling point | 210 to 220 °C (410 to 428 °F; 483 to 493 K) 0.01 Torr |
| 0.0013 g/100 mL | |
| Solubility | |
| Vapor pressure | 1.32×10−8 mm Hg at 25 °C[2] |
| UV-vis (λmax) | 280 nm |
| Structure | |
| Monoclinic | |
| Pharmacology | |
| M02AB01 (WHO) N01BX04 (WHO) | |
| License data | |
| Legal status | |
| Hazards | |
| GHS labelling: | |
   
| |
| Danger | |
| H301, H302, H315, H318 | |
| P264, P270, P280, P301+P310, P301+P312, P302+P352, P305+P351+P338, P310, P321, P330, P332+P313, P362, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | [2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Capsaicin | |
|---|---|
| Heat | Above peak (pure capsaicin is toxic)[2] |
| Scoville scale | 16,000,000[5] SHU |
Capsaicin (8-methyl-N-vanillyl-6-nonenamide) (/kæpˈseɪsɪn/ or /kæpˈseɪəsɪn/) is an active component of chili peppers, which are plants belonging to the genus Capsicum. It is a chemical irritant and neurotoxin[6] for mammals, including humans, and produces a sensation of burning in any tissue with which it comes into contact. Capsaicin and several related amides (capsaicinoids) are produced as secondary metabolites by chili peppers, likely as deterrents against certain mammals and fungi.[7] Pure capsaicin is a hydrophobic, colorless, highly pungent (i.e., spicy) crystalline solid.[2]
- ^ "Capsaicin". ChemSpider, Royal Society of Chemistry, Cambridge, UK. 2018. Retrieved 9 June 2018.
- ^ a b c d "Capsaicin". PubChem, US National Library of Medicine. 27 May 2023. Retrieved 1 June 2023.
- ^ "Qutenza- capsaicin kit". DailyMed. 10 January 2023. Retrieved 22 February 2023.
- ^ "Drug Approval Package: Qutenza (capsaicin) NDA #022395". U.S. Food and Drug Administration (FDA). 3 October 2013. Retrieved 22 February 2023.
- ^ Govindarajan VS, Sathyanarayana MN (1991). "Capsicum--production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences". Critical Reviews in Food Science and Nutrition. 29 (6): 435–474. doi:10.1080/10408399109527536. PMID 2039598.
- ^ Ritter S, Dinh TT (June 1990). "Capsaicin-induced neuronal degeneration in the brain and retina of preweanling rats". The Journal of Comparative Neurology. 296 (3): 447–461. doi:10.1002/cne.902960310. PMID 2358547. S2CID 5468197.
- ^ "What Made Chili Peppers So Spicy?". Talk of the Nation. 15 August 2008.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
