
Back Koolstofsuboksied Afrikaans تحت أكسيد الكربون Arabic کربون سابوکسید AZB Suboxid uhlíku Czech Kohlenstoffsuboxid German Υποξείδιο του άνθρακα Greek Dióxido de tricarbono Spanish کربن سابوکسید Persian Hiilisuboksidi Finnish Suboxyde de carbone French
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Propa-1,2-diene-1,3-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | Carbon+suboxide |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3O2 | |
| Molar mass | 68.031 g·mol−1 |
| Appearance | colorless gas |
| Odor | strong, pungent odor |
| Density | 3.0 kg/m3, gas[1]
1.114 g/cm3, liquid[2] |
| Melting point | −111.3 °C (−168.3 °F; 161.8 K) |
| Boiling point | 6.8 °C (44.2 °F; 279.9 K) |
| reacts | |
| Solubility | soluble in 1,4-dioxane, ether, xylene, CS2, tetrahydrofuran |
Refractive index (nD)
|
1.4538 (6 °C) |
| 0 D | |
| Structure | |
| rhombic | |
| quasilinear (phase dependent) | |
| Thermochemistry | |
Heat capacity (C)
|
66.99 J/mol K |
Std molar
entropy (S⦵298) |
276.1 J/mol K |
Std enthalpy of
formation (ΔfH⦵298) |
−93.6 kJ/mol |
| Related compounds | |
Related oxides
|
carbon dioxide carbon monoxide dicarbon monoxide |
Related compounds
|
carbon subsulfide carbon subnitride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
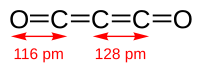
Carbon suboxide, or tricarbon dioxide, is an organic, oxygen-containing chemical compound with formula C3O2 and structure O=C=C=C=O. Its four cumulative double bonds make it a cumulene. It is one of the stable members of the series of linear oxocarbons O=Cn=O, which also includes carbon dioxide (CO2) and pentacarbon dioxide (C5O2). Although if carefully purified it can exist at room temperature in the dark without decomposing, it will polymerize under certain conditions.
The substance was discovered in 1873 by Benjamin Brodie by subjecting carbon monoxide to an electric current. He claimed that the product was part of a series of "oxycarbons" with formulas Cx+1Ox, namely C2O, C3O2, C4O3, C5O4, …, and to have identified the last two;[3][4] however, only C3O2 is known. In 1891 Marcellin Berthelot observed that heating pure carbon monoxide at about 550 °C created small amounts of carbon dioxide but no trace of carbon, and assumed that a carbon-rich oxide was created instead, which he named "sub-oxide". He assumed it was the same product obtained by electric discharge and proposed the formula C2O.[5] Otto Diels later stated that the more organic names dicarbonylmethane and dioxallene were also correct.
It is commonly described as an oily liquid or gas at room temperature with an extremely noxious odor.[6]
- ^ "Carbon Suboxide". WebElements Periodic Table. Retrieved 19 Feb 2019.
- ^ Weast RC, Astle MJ, eds. (1983). CRC Handbook of Chemistry and Physics (64th ed.). Boca Raton: CRC Press. p. B-82. ISBN 9780849304637.
- ^ Brodie BC (1873). "Note on the Synthesis of Marsh-Gas and Formic Acid, and on the Electric Decomposition of Carbonic Oxide". Proc. R. Soc. Lond. 21 (139–147): 245–247. doi:10.1098/rspl.1872.0052. JSTOR 113037.
When pure and dry carbonic oxide [=carbon monoxide] is circulated through the induction-tube, and there submitted to the action of electricity, a decomposition of the gas occurs [...] Carbonic acid [=carbon dioxide] is formed, and simultaneously with its formation a solid deposit may be observed in the induction-tube. This deposit appears as a transparent film of a red-brown color, lining the walls of the tube. It is perfectly soluble in water, which is strongly colored by it. The solution has an intensely acid reaction. The solid deposit, in the dry condition before it has been in contact with the water, is an oxide of carbon.
- ^ Brodie BC (1873). "Ueber eine Synthese von Sumpfgas und Ameisensäure und die electrische Zersetzung des Kohlenoxyds". Liebigs Ann. 169 (1–2): 270–271. doi:10.1002/jlac.18731690119.
- ^ Berthelot M (1891). "Action de la chaleur sur l'oxyde de carbone". Annales de Chimie et de Physique. 6 (24): 126–132. Archived from the original on 17 February 2012. Retrieved 21 Feb 2007.
- ^ Reyerson LH, Kobe K (1930). "Carbon Suboxide". Chem. Rev. 7 (4): 479–492. doi:10.1021/cr60028a002.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search