
Back Sitochroom P450 Afrikaans سيتوكروم بي450 Arabic Citohrom P450 BS Citocrom p450 Catalan Cytochrom P450 Czech Cytochrom P450 German Κυτόχρωμα P450 Greek Citokromo p450 Esperanto Citocromo P450 Spanish Tsütokroom P450 Estonian
| Cytochrome P450 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
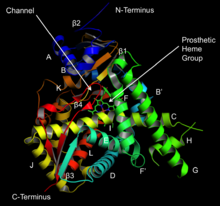 Structure of lanosterol 14α-demethylase (CYP51) | |||||||||
| Identifiers | |||||||||
| Symbol | p450 | ||||||||
| Pfam | PF00067 | ||||||||
| InterPro | IPR001128 | ||||||||
| PROSITE | PDOC00081 | ||||||||
| SCOP2 | 2cpp / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 39 | ||||||||
| OPM protein | 2bdm | ||||||||
| CDD | cd00302 | ||||||||
| Membranome | 265 | ||||||||
| |||||||||
Cytochromes P450 (P450s or CYPs) are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases.[1] However, they are not omnipresent; for example, they have not been found in Escherichia coli.[2] In mammals, these enzymes oxidize steroids, fatty acids, xenobiotics, and participate in many biosyntheses.[1] By hydroxylation, CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted.
P450s are, in general, the terminal oxidase enzymes in electron transfer chains, broadly categorized as P450-containing systems. The term "P450" is derived from the spectrophotometric peak at the wavelength of the absorption maximum of the enzyme (450 nm) when it is in the reduced state and complexed with carbon monoxide. Most P450s require a protein partner to deliver one or more electrons to reduce the iron (and eventually molecular oxygen).
- ^ a b "Cytochrome P450". InterPro.
- ^ Danielson PB (December 2002). "The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans". Current Drug Metabolism. 3 (6): 561–597. doi:10.2174/1389200023337054. PMID 12369887.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search