
Back فرضية إرغوديك Arabic Ergodenhypothese German Hipótesis de ergodicidad Spanish Ergodinen hypoteesi Finnish Hypothèse ergodique French Ipotesi ergodica Italian 에르고딕 가설 Korean Hipoteza ergodyczna Polish Hipótese de ergodicidade Portuguese Эргодическая гипотеза Russian
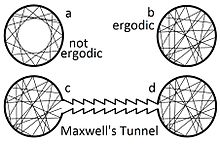

In physics and thermodynamics, the ergodic hypothesis[1] says that, over long periods of time, the time spent by a system in some region of the phase space of microstates with the same energy is proportional to the volume of this region, i.e., that all accessible microstates are equiprobable over a long period of time.
Liouville's theorem states that, for a Hamiltonian system, the local density of microstates following a particle path through phase space is constant as viewed by an observer moving with the ensemble (i.e., the convective time derivative is zero). Thus, if the microstates are uniformly distributed in phase space initially, they will remain so at all times. But Liouville's theorem does not imply that the ergodic hypothesis holds for all Hamiltonian systems.
The ergodic hypothesis is often assumed in the statistical analysis of computational physics. The analyst would assume that the average of a process parameter over time and the average over the statistical ensemble are the same. This assumption—that it is as good to simulate a system over a long time as it is to make many independent realizations of the same system—is not always correct. (See, for example, the Fermi–Pasta–Ulam–Tsingou experiment of 1953.)
Assumption of the ergodic hypothesis allows proof that certain types of perpetual motion machines of the second kind are impossible.
Systems that are ergodic are said to have the property of ergodicity; a broad range of systems in geometry, physics, and probability are ergodic. Ergodic systems are studied in ergodic theory.
- ^ Originally due to L. Boltzmann. See part 2 of Vorlesungen über Gastheorie. Leipzig: J. A. Barth. 1898. OCLC 01712811. ('Ergoden' on p.89 in the 1923 reprint.) It was used to prove equipartition of energy in the kinetic theory of gases.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search