| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
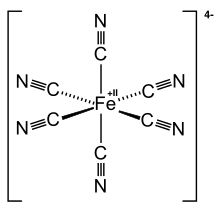
| C6FeN64− | |
| Molar mass | 211.955 g·mol−1 |
| Related compounds | |
Related compounds
|
Ferricyanide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ferrocyanide is the name of the anion [Fe(CN)6]4−. Salts of this coordination complex give yellow solutions. It is usually available as the salt potassium ferrocyanide, which has the formula K4Fe(CN)6. [Fe(CN)6]4− is a diamagnetic species, featuring low-spin iron(II) center in an octahedral ligand environment. Although many salts of cyanide are highly toxic, ferro- and ferricyanides are less toxic because they tend not to release free cyanide.[1] It is of commercial interest as a precursor to the pigment Prussian blue and, as its potassium salt, an anticaking agent.[2]
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- ^ Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M.; Kellens, R.; Reddy, J.; Steier, N.; Hasenpusch, W. (2011). "Cyano Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_159.pub3. ISBN 978-3527306732.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
