Back Glisien Afrikaans جلايسين Arabic Aminosirkə turşusu Azerbaijani قلیسین AZB Гліцын Byelorussian Глицин Bulgarian গ্লাইসিন Bengali/Bangla Glicin BS Glicina Catalan Glycin Czech
| |||
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Glycine
| |||
| Systematic IUPAC name
Aminoacetic acid[2] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | Gly, G | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.248 | ||
| EC Number |
| ||
| E number | E640 (flavour enhancer) | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H5NO2 | |||
| Molar mass | 75.067 g·mol−1 | ||
| Appearance | White solid | ||
| Density | 1.1607 g/cm3[3] | ||
| Melting point | 233 °C (451 °F; 506 K) (decomposition) | ||
| 249.9 g/L (25 °C)[4] | |||
| Solubility | soluble in pyridine sparingly soluble in ethanol insoluble in ether | ||
| Acidity (pKa) | 2.34 (carboxyl), 9.6 (amino)[5] | ||
| -40.3·10−6 cm3/mol | |||
| Pharmacology | |||
| B05CX03 (WHO) | |||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
2600 mg/kg (mouse, oral) | ||
| Supplementary data page | |||
| Glycine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
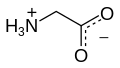
Glycine (symbol Gly or G;[6] /ˈɡlaɪsiːn/ )[7] is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable). In the gas phase, it is a molecule with the chemical formula NH2‐CH2‐COOH. In solution or in the solid, glycine exists as the zwitterion. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to the "flexibility" caused by such a small R group. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a Clostridium tetani infection) can cause spastic paralysis due to uninhibited muscle contraction.
It is the only achiral proteinogenic amino acid. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom.
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, ISBN 091191028X, 4386
- ^ pubchem.ncbi.nlm.nih.gov/compound/750#section=IUPAC-Name&fullscreen=true
- ^ Handbook of Chemistry and Physics, CRC Press, 59th edition, 1978
- ^ "Solubilities and densities". Prowl.rockefeller.edu. Archived from the original on September 12, 2017. Retrieved November 13, 2013.
- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ^ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on October 9, 2008. Retrieved March 5, 2018.
- ^ "Glycine | Definition of glycine in English by Oxford Dictionaries". Archived from the original on January 29, 2018.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search