
Back تحفيز غير متجانس Arabic Catàlisi heterogènia Catalan Heterogenní katalýza Czech Heterogen katalyse Danish Heterogene Katalyse German Catálisis heterogénea Spanish Heterogeenne katalüüs Estonian Katalisi heterogeneo Basque کاتالیز ناهمگن Persian Heterogeeninen katalyysi Finnish
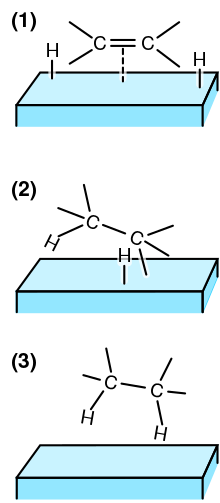
Heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products.[1] The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures (e.g. oil and water), or anywhere an interface is present.
Heterogeneous catalysis typically involves solid phase catalysts and gas phase reactants.[2] In this case, there is a cycle of molecular adsorption, reaction, and desorption occurring at the catalyst surface. Thermodynamics, mass transfer, and heat transfer influence the rate (kinetics) of reaction.
Heterogeneous catalysis is very important because it enables faster, large-scale production and the selective product formation.[3] Approximately 35% of the world's GDP is influenced by catalysis.[4] The production of 90% of chemicals (by volume) is assisted by solid catalysts.[2] The chemical and energy industries rely heavily on heterogeneous catalysis. For example, the Haber–Bosch process uses metal-based catalysts in the synthesis of ammonia, an important component in fertilizer; 144 million tons of ammonia were produced in 2016.[5]
- ^ Schlögl, Robert (9 March 2015). "Heterogeneous Catalysis". Angewandte Chemie International Edition. 54 (11): 3465–3520. doi:10.1002/anie.201410738. hdl:11858/00-001M-0000-0025-0A33-6. PMID 25693734.
- ^ a b Rothenberg, Gadi (17 March 2008). Catalysis : concepts and green applications. Weinheim [Germany]: Wiley-VCH. ISBN 9783527318247. OCLC 213106542.
- ^ Information., Lawrence Berkeley National Laboratory. United States. Department of Energy. Office of Scientific and Technical (2003). "The impact of nanoscience on heterogeneous catalysis". Science. 299 (5613). Lawrence Berkeley National Laboratory: 1688–1691. Bibcode:2003Sci...299.1688B. doi:10.1126/science.1083671. OCLC 727328504. PMID 12637733. S2CID 35805920.
- ^ Ma, Zhen; Zaera, Francisco (2006-03-15), "Heterogeneous Catalysis by Metals", in King, R. Bruce; Crabtree, Robert H.; Lukehart, Charles M.; Atwood, David A. (eds.), Encyclopedia of Inorganic Chemistry, John Wiley & Sons, Ltd, doi:10.1002/0470862106.ia084, ISBN 9780470860786
- ^ "United States Geological Survey, Mineral Commodity Summaries" (PDF). USGS. January 2018.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search