
Back Waterstofbinding Afrikaans Vinclo d'hidrocheno AN رابطة هيدروجينية Arabic Fuercia per ponte d'hidróxenu AST Hidrogen rabitəsi Azerbaijani Вадародная сувязь Byelorussian Водородна връзка Bulgarian হাইড্রোজেন বন্ধন Bengali/Bangla Vodikova veza BS Enllaç per pont d'hidrogen Catalan
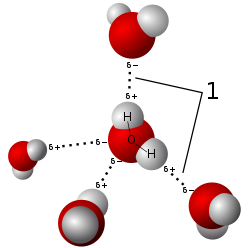

In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, covalently bonded to a more electronegative donor atom or group (Dn), interacts with another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Unlike simple dipole–dipole interactions, hydrogen bonding arises from charge transfer (nB → σ*AH), orbital interactions, and quantum mechanical delocalization, making it a resonance-assisted interaction rather than a mere electrostatic attraction.[5]
The general notation for hydrogen bonding is Dn−H···Ac, where the solid line represents a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond.[6] The most frequent donor and acceptor atoms are nitrogen (N), oxygen (O), and fluorine (F), due to their high electronegativity and ability to engage in stronger hydrogen bonding.
The term "hydrogen bond" is generally used for well-defined, localized interactions with significant charge transfer and orbital overlap, such as those in DNA base pairing or ice. In contrast, "hydrogen-bonding interactions" is a broader term used when the interaction is weaker, more dynamic, or delocalized, such as in liquid water, supramolecular assemblies (e.g.: lipid membranes, protein-protein interactions), or weak C-H···O interactions. This distinction is particularly relevant in structural biology, materials science, and computational chemistry, where hydrogen bonding spans a continuum from weak van der Waals-like interactions to nearly covalent bonding.[5]
Hydrogen bonding can occur between separate molecules (intermolecular) or within different parts of the same molecule (intramolecular).[7][8][9][10] Its strength varies considerably, depending on geometry, environment, and the donor-acceptor pair, typically ranging from 1 to 40 kcal/mol.[11] This places hydrogen bonds stronger than van der Waals interactions but generally weaker than covalent or ionic bonds.
Hydrogen bonding plays a fundamental role in chemistry, biology, and materials science. It is responsible for the anomalously high boiling point of water, the stabilization of protein and nucleic acid structures, and key properties of materials like paper, wool, and hydrogels. In biological systems, hydrogen bonds mediate molecular recognition, enzyme catalysis, and DNA replication, while in materials science, they contribute to self-assembly, adhesion, and supramolecular organization.
- ^ Sweetman, A. M.; Jarvis, S. P.; Sang, Hongqian; Lekkas, I.; Rahe, P.; Wang, Yu; Wang, Jianbo; Champness, N.R.; Kantorovich, L.; Moriarty, P. (2014). "Mapping the force field of a hydrogen-bonded assembly". Nature Communications. 5: 3931. Bibcode:2014NatCo...5.3931S. doi:10.1038/ncomms4931. PMC 4050271. PMID 24875276.
- ^ Hapala, Prokop; Kichin, Georgy; Wagner, Christian; Tautz, F. Stefan; Temirov, Ruslan; Jelínek, Pavel (2014-08-19). "Mechanism of high-resolution STM/AFM imaging with functionalized tips". Physical Review B. 90 (8): 085421. arXiv:1406.3562. Bibcode:2014PhRvB..90h5421H. doi:10.1103/PhysRevB.90.085421. S2CID 53610973.
- ^ De Luca, S.; Chen, F.; Seal, P.; Stenzel, M. H.; Smith, S. C. (2017). "Binding and Release between Polymeric Carrier and Protein Drug: pH-Mediated Interplay of Coulomb Forces, Hydrogen Bonding, van der Waals Interactions, and Entropy". Biomacromolecules. 18 (11): 3665–3677. doi:10.1021/acs.biomac.7b00657. hdl:1959.4/unsworks_55160. PMID 28880549.
- ^ Hämäläinen, Sampsa K.; van der Heijden, Nadine; van der Lit, Joost; den Hartog, Stephan; Liljeroth, Peter; Swart, Ingmar (2014-10-31). "Intermolecular Contrast in Atomic Force Microscopy Images without Intermolecular Bonds". Physical Review Letters. 113 (18): 186102. arXiv:1410.1933. Bibcode:2014PhRvL.113r6102H. doi:10.1103/PhysRevLett.113.186102. hdl:1874/307996. PMID 25396382. S2CID 8309018. Archived from the original on 2018-01-20. Retrieved 2017-08-30.
- ^ a b Weinhold, Frank; Klein, Roger A. (2014-07-08). "What is a hydrogen bond? Resonance covalency in the supramolecular domain". Chemistry Education Research and Practice. 15 (3): 276–285. doi:10.1039/C4RP00030G. ISSN 1756-1108.
- ^ Arunan, Elangannan; Desiraju, Gautam R.; Klein, Roger A.; Sadlej, Joanna; Scheiner, Steve; Alkorta, Ibon; Clary, David C.; Crabtree, Robert H.; Dannenberg, Joseph J. (2011-07-08). "Definition of the hydrogen bond (IUPAC Recommendations 2011)". Pure and Applied Chemistry. 83 (8): 1637–1641. doi:10.1351/PAC-REC-10-01-02. ISSN 1365-3075. S2CID 97688573.
- ^ Pimentel, G. The Hydrogen Bond Franklin Classics, 2018), ISBN 0343171600
- ^ Jeffrey, G. A.; An introduction to hydrogen bonding; Oxford university press New York, 1997. ISBN 0195095499
- ^ Jeffrey, G. A.; Saenger, W. Hydrogen bonding in biological structures; Springer: Berlin, 1994, 2012 Springer; ISBN 3540579036
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "hydrogen bond". doi:10.1351/goldbook.H02899
- ^ Steiner, Thomas (2002). "The Hydrogen Bond in the Solid State". Angew. Chem. Int. Ed. 41 (1): 48–76. doi:10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-U. PMID 12491444.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search