
Back كلوريد إنديوم ثلاثي Arabic کولورید ایندیوم (III) AZB Indium(III)-chlorid German کلرید ایندیم (III) Persian Indium(III)kloridi Finnish Chlorure d'indium(III) French 塩化インジウム(III) Japanese Indium(III)chloride Dutch Хлорид индия(III) Russian Indijum(III) hlorid Serbo-Croatian
| |
 Tetrahydrate
| |
| Names | |
|---|---|
| Other names
Indium chloride
Indium trichloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.027 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3260 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| InCl3 | |
| Molar mass | 221.18 g/mol |
| Appearance | white flakes |
| Density | 3.46 g/cm3 |
| Melting point | 586 °C (1,087 °F; 859 K) |
| Boiling point | 800 °C (1,470 °F; 1,070 K) |
| 195 g/100 mL, exothermic | |
| Solubility in other solvents | THF, Ethanol |
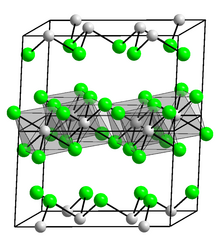
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Corrosive |
| GHS labelling: | |
  [1] [1]
| |
| Danger[1] | |
| H302, H314[1] | |
| P260, P301+P330+P331, P303+P361+P353, P305+P351+P338, P405, P501[1] | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External SDS |
| Related compounds | |
Other anions
|
Indium(III) fluoride Indium(III) bromide Indium(III) iodide |
Other cations
|
Aluminium chloride Gallium trichloride Thallium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Indium(III) chloride is the chemical compound with the formula InCl3 which forms a tetrahydrate. This salt is a white, flaky solid with applications in organic synthesis as a Lewis acid. It is also the most available soluble derivative of indium.[2] This is one of three known indium chlorides.
- ^ a b c d "Indium(III) Chloride". American Elements. Retrieved May 15, 2019.
- ^ Araki, S.; Hirashita, T. "Indium trichloride" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
