
Back Isòtops del tal·li Catalan Izotopy thallia Czech Талли изотопĕсем CV Ισότοπα του θαλλίου Greek Anexo:Isótopos de talio Spanish ایزوتوپهای تالیم Persian Isotopes du thallium French A tallium izotópjai Hungarian Isotop talium ID タリウムの同位体 Japanese
| |||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Tl) | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
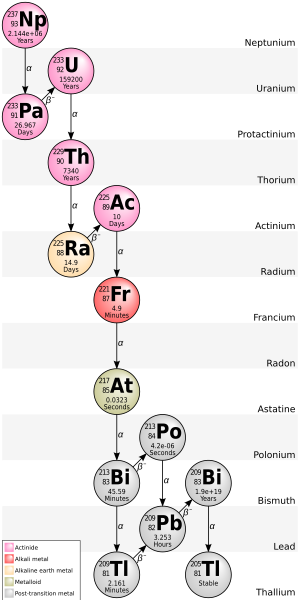
Thallium (81Tl) has 41 isotopes with atomic masses that range from 176 to 216. 203Tl and 205Tl are the only stable isotopes and 204Tl is the most stable radioisotope with a half-life of 3.78 years. 207Tl, with a half-life of 4.77 minutes, has the longest half-life of naturally occurring Tl radioisotopes. All isotopes of thallium are either radioactive or observationally stable, meaning that they are predicted to be radioactive but no actual decay has been observed.
Thallium-202 (half-life 12.23 days) can be made in a cyclotron[4] while thallium-204 (half-life 3.78 years) is made by the neutron activation of stable thallium in a nuclear reactor.[5]
In the fully ionized state, the isotope 205Tl becomes beta-radioactive, decaying to 205Pb,[6] but 203Tl remains stable.
205Tl is the decay product of bismuth-209, an isotope that was once thought to be stable but is now known to undergo alpha decay with an extremely long half-life of 2.01×1019 y.[7] 205Tl is at the end of the neptunium series decay chain.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ "Standard Atomic Weights: Thallium". CIAAW. 2009.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ "Thallium Research". doe.gov. Department of Energy. Archived from the original on 2006-12-09. Retrieved 23 March 2018.
- ^ Manual for reactor produced radioisotopes from the International Atomic Energy Agency
- ^ "Bound-state beta decay of highly ionized atoms" (PDF). Archived from the original (PDF) on October 29, 2013. Retrieved June 9, 2013.
- ^ Marcillac, P.; Coron, N.; Dambier, G.; et al. (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201. S2CID 4415582.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search