
Back Magnesiumkarbonaat Afrikaans كربونات المغنيسيوم Arabic منیزیوم کربونات AZB ম্যাগনেসিয়াম কার্বনেট Bengali/Bangla Magnezij-karbonat BS Uhličitan hořečnatý Czech Magnesiumcarbonat Danish Magnesiumcarbonat German Ανθρακικό μαγνήσιο Greek Magnezia karbonato Esperanto
| |
| |

| |
| Names | |
|---|---|
| Other names | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.106 |
| E number | E504(i) (acidity regulators, ...) |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| MgCO3 | |
| Molar mass | 84.3139 g/mol (anhydrous) |
| Appearance | Colourless crystals or white solid Hygroscopic |
| Odor | Odorless |
| Density | 2.958 g/cm3 (anhydrous) 2.825 g/cm3 (dihydrate) 1.837 g/cm3 (trihydrate) 1.73 g/cm3 (pentahydrate) |
| Melting point | 350 °C (662 °F; 623 K) decomposes (anhydrous) 165 °C (329 °F; 438 K) (trihydrate) |
| Anhydrous: 0.0139 g/100 ml (25 °C) 0.0063 g/100 ml (100 °C)[1] | |
Solubility product (Ksp)
|
10−7.8[2] |
| Solubility | Soluble in acid, aqueous CO2 Insoluble in acetone, ammonia |
| −32.4·10−6 cm3/mol | |
Refractive index (nD)
|
1.717 (anhydrous) 1.458 (dihydrate) 1.412 (trihydrate) |
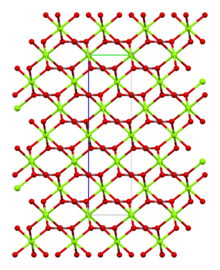
| Structure | |
| Trigonal | |
| R3c, No. 167[3] | |
| Thermochemistry | |
Heat capacity (C)
|
75.6 J/mol·K[1] |
Std molar
entropy (S⦵298) |
65.7 J/mol·K[1][4] |
Std enthalpy of
formation (ΔfH⦵298) |
−1113 kJ/mol[4] |
Gibbs free energy (ΔfG⦵)
|
−1029.3 kJ/mol[1] |
| Pharmacology | |
| A02AA01 (WHO) A06AD01 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
|
| Safety data sheet (SDS) | ICSC 0969 |
| Related compounds | |
Other anions
|
Magnesium bicarbonate |
Other cations
|
Beryllium carbonate Calcium carbonate Strontium carbonate Barium carbonate Radium carbonate |
Related compounds
|
Artinite Hydromagnesite Dypingite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
You can help expand this article with text translated from the corresponding article in German. (December 2018) Click [show] for important translation instructions.
|
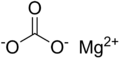
Magnesium carbonate, MgCO3 (archaic name magnesia alba), is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.
- ^ a b c d "Magnesium carbonate".
- ^ Bénézeth, Pascale; Saldi, Giuseppe D.; Dandurand, Jean-Louis; Schott, Jacques (2011). "Experimental determination of the solubility product of magnesite at 50 to 200 °C". Chemical Geology. 286 (1–2): 21–31. Bibcode:2011ChGeo.286...21B. doi:10.1016/j.chemgeo.2011.04.016.
- ^ Cite error: The named reference
Rosswas invoked but never defined (see the help page). - ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0373". National Institute for Occupational Safety and Health (NIOSH).
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
