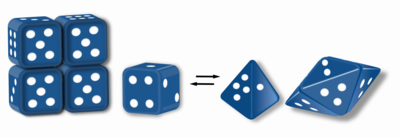
Morpheeins are proteins that can form two or more different homo-oligomers (morpheein forms), but must come apart and change shape to convert between forms. The alternate shape may reassemble to a different oligomer. The shape of the subunit dictates which oligomer is formed.[1][2] Each oligomer has a finite number of subunits (stoichiometry). Morpheeins can interconvert between forms under physiological conditions and can exist as an equilibrium of different oligomers. These oligomers are physiologically relevant and are not misfolded protein; this distinguishes morpheeins from prions and amyloid. The different oligomers have distinct functionality. Interconversion of morpheein forms can be a structural basis for allosteric regulation, an idea noted many years ago,[3][4] and later revived.[1][2][5][6] A mutation that shifts the normal equilibrium of morpheein forms can serve as the basis for a conformational disease.[7] Features of morpheeins can be exploited for drug discovery.[1][5][8] The dice image (Fig 1) represents a morpheein equilibrium containing two different monomeric shapes that dictate assembly to a tetramer or a pentamer. The one protein that is established to function as a morpheein is porphobilinogen synthase,[2][9][10] though there are suggestions throughout the literature that other proteins may function as morpheeins (for more information see "Table of Putative Morpheeins" below).
- ^ a b c Jaffe, Eileen K. (2005). "Morpheeins – a new structural paradigm for allosteric regulation". Trends in Biochemical Sciences. 30 (9): 490–7. doi:10.1016/j.tibs.2005.07.003. PMID 16023348.
- ^ a b c Breinig, Sabine; Kervinen, Jukka; Stith, Linda; Wasson, Andrew S; Fairman, Robert; Wlodawer, Alexander; Zdanov, Alexander; Jaffe, Eileen K (2003). "Control of tetrapyrrole biosynthesis by alternate quaternary forms of porphobilinogen synthase". Nature Structural Biology. 10 (9): 757–63. doi:10.1038/nsb963. PMID 12897770. S2CID 24188785.
- ^ Cite error: The named reference
Frieden67was invoked but never defined (see the help page). - ^ Cite error: The named reference
Nichol67was invoked but never defined (see the help page). - ^ a b Lawrence, Sarah H.; Ramirez, Ursula D.; Tang, Lei; Fazliyez, Farit; Kundrat, Lenka; Markham, George D.; Jaffe, Eileen K. (2008). "Shape Shifting Leads to Small-Molecule Allosteric Drug Discovery". Chemistry & Biology. 15 (6): 586–96. doi:10.1016/j.chembiol.2008.04.012. PMC 2703447. PMID 18559269.
- ^ Selwood, Trevor; Jaffe, Eileen K. (2012). "Dynamic dissociating homo-oligomers and the control of protein function". Archives of Biochemistry and Biophysics. 519 (2): 131–43. doi:10.1016/j.abb.2011.11.020. PMC 3298769. PMID 22182754.
- ^ Jaffe, Eileen K.; Stith, Linda (2007). "ALAD Porphyria is a Conformational Disease". The American Journal of Human Genetics. 80 (2): 329–37. doi:10.1086/511444. PMC 1785348. PMID 17236137.
- ^ Jaffe, Eileen K. (2010). "Morpheeins – A New Pathway For Allosteric Drug Discovery". The Open Conference Proceedings Journal. 1: 1–6. doi:10.2174/2210289201001010001 (inactive 2024-03-28). PMC 3107518. PMID 21643557.
{{cite journal}}: CS1 maint: DOI inactive as of March 2024 (link) - ^ Tang, L.; Stith, L; Jaffe, EK (2005). "Substrate-induced Interconversion of Protein Quaternary Structure Isoforms". Journal of Biological Chemistry. 280 (16): 15786–93. doi:10.1074/jbc.M500218200. PMID 15710608.
- ^ Jaffe, Eileen K.; Lawrence, Sarah H. (2012). "Allostery and the dynamic oligomerization of porphobilinogen synthase". Archives of Biochemistry and Biophysics. 519 (2): 144–53. doi:10.1016/j.abb.2011.10.010. PMC 3291741. PMID 22037356.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
