
Back Poro nucleyar AN مسام نووي Arabic Nüvə məsaməsi Azerbaijani Jedrova pora BS Porus nuclear Catalan Jaderný pór Czech Kernpore German Πυρηνικός πόρος Greek Nuklea poro Esperanto Poro nuclear Spanish
| Nuclear pore complex | |
|---|---|
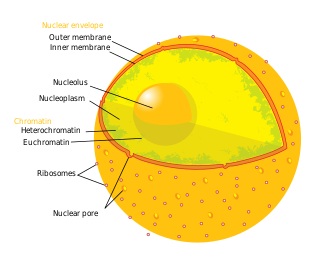 Diagram of the human cell nucleus with nuclear pores. | |
 Schematic diagram of a nuclear pore complex within the nuclear envelope (1) with the outer ring (2), spokes (3), basket (4), and filaments (5). | |
| Details | |
| Identifiers | |
| Latin | porus nuclearis |
| MeSH | D022022 |
| TH | H1.00.01.2.01005 |
| FMA | 63148 |
| Anatomical terminology | |
The nuclear pore complex (NPC), is a large protein complex giving rise to the nuclear pore. A great number of nuclear pores are studded throughout the nuclear envelope that surrounds the eukaryote cell nucleus. The pores enable the nuclear transport of macromolecules between the nucleoplasm of the nucleus and the cytoplasm of the cell. Small molecules can easily diffuse through the pores.[1] Nuclear transport includes the transportation of RNA and ribosomal proteins from the nucleus to the cytoplasm, and the transport of proteins (such as DNA polymerase and lamins), carbohydrates, signaling molecules, and lipids into the nucleus. Each nuclear pore complex can actively mediate up to 1000 translocations per second.
The nuclear pore complex consists predominantly of a family of proteins known as nucleoporins (Nups). Each pore complex in the human cell nucleus is composed of about 1,000 individual protein molecules, from an evolutionarily conserved set of 35 distinct nucleoporins.[2] The conserved sequences that code for nucleoporins regulate molecular transport through the nuclear pore.[3][4] Nucleoporin-mediated transport does not entail direct energy expenditure but instead relies on concentration gradients associated with the RAN cycle (Ras-related nuclear protein cycle). In 2022 around 90% of the structure of the human NPC was elucidated in an open and a closed conformation, and published in a special issue of Science, featured on the cover.[5][6][7] In 2024 the structure of the nuclear basket was solved, finalising the completion of the structure of the nuclear pore complex.[8]
About half of the nucleoporins encompass solenoid protein domains, such as alpha solenoids or beta-propeller folds, and occasionally both as separate structural domains. Conversely, the remaining nucleoporins exhibit characteristics of "natively unfolded" or intrinsically disordered proteins, characterized by high flexibility and a lack of ordered tertiary structure. These disordered proteins, referred to as FG nucleoporins (FG-Nups), contain multiple phenylalanine–glycine repeats (FG repeats) in their amino acid sequences.[9] FG-Nups is one of three main types of nucleoporins found in the NPC. The other two are the transmembrane Nups and the scaffold Nups. The transmembrane Nups are made up of transmembrane alpha helices and play a vital part in anchoring the NPC to the nuclear envelope. The scaffold Nups are made up of alpha solenoid and beta-propeller folds, and create the structural framework of NPCs.[10]
The count of nuclear pore complexes varies across cell types and different stages of the cell's life cycle, with approximately 1,000 NPCs typically found in vertebrate cells.[11] The human nuclear pore complex is a substantial structure, with a molecular weight of 120 megadaltons (MDa).[12] Each NPC comprises eight protein subunits encircling the actual pore, forming the outer ring. Additionally, these subunits project a spoke-shaped protein over the pore channel. The central region of the pore may exhibit a plug-like structure; however, its precise nature remains unknown, and it is yet undetermined whether it represents an actual plug or merely cargo transiently caught in transit.
- ^ Alberts B, Johnson AD, Morgan D, Roberts K, Walter P, Raff MC, et al. (2014). Molecular Biology of the Cell. Garland Science, Taylor and Francis Group. p. 179. ISBN 978-0-393-53696-6.
- ^ Lin DH, Stuwe T, Schilbach S, Rundlet EJ, Perriches T, Mobbs G, et al. (April 15, 2016). "Architecture of the symmetric core of the nuclear pore". Science. 352 (6283): aaf1015. doi:10.1126/science.aaf1015. PMC 5207208. PMID 27081075.
- ^ Peyro M, Soheilypour M, Lee BL, Mofrad MR (November 2015). "Evolutionarily Conserved Sequence Features Regulate the Formation of the FG Network at the Center of the Nuclear Pore Complex". Scientific Reports. 5: 15795. Bibcode:2015NatSR...515795P. doi:10.1038/srep15795. PMC 4635341. PMID 26541386.
- ^ Ando D, Colvin M, Rexach M, Gopinathan A (September 16, 2013). "Physical motif clustering within intrinsically disordered nucleoporin sequences reveals universal functional features". PLOS ONE. 8 (9): e73831. Bibcode:2013PLoSO...873831A. doi:10.1371/journal.pone.0073831. PMC 3774778. PMID 24066078.
- ^ Mosalaganti S, Obarska-Kosinska A, Siggel M, Taniguchi R, Turoňová B, Zimmerli CE, et al. (June 10, 2022). "AI-based structure prediction empowers integrative structural analysis of human nuclear pores". Science. 376 (6598): eabm9506. doi:10.1126/science.abm9506. PMID 35679397.
- ^ Petrovic S, Samanta D, Perriches T, Bley CJ, Thierbach K, Brown B, et al. (June 10, 2022). "Architecture of the linker-scaffold in the nuclear pore". Science. 376 (6598): eabm9798. doi:10.1126/science.abm9798. PMC 9867570. PMID 35679425.
- ^ Bley CJ, Nie S, Mobbs GW, Petrovic S, Gres AT, Liu X, et al. (June 10, 2022). "Architecture of the cytoplasmic face of the nuclear pore". Science. 376 (6598): eabm9129. doi:10.1126/science.abm9129. PMC 9348906. PMID 35679405.
- ^ Singh D, Soni N, Hutchings J, Echeverria I, Shaikh F, Duquette M, et al. (September 2024). "The molecular architecture of the nuclear basket". Cell. 187 (19): 5267–5281.e13. doi:10.1016/j.cell.2024.07.020. PMC 11416316. PMID 39127037.
- ^ Field MC, Rout MP (April 3, 2019). "Pore timing: the evolutionary origins of the nucleus and nuclear pore complex". F1000Research. 8: 369. doi:10.12688/f1000research.16402.1. PMC 6449795. PMID 31001417.
- ^ Nag N, Sasidharan S, Uversky VN, Saudagar P, Tripathi T (April 2022). "Phase separation of FG-nucleoporins in nuclear pore complexes". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1869 (4): 119205. doi:10.1016/j.bbamcr.2021.119205. PMID 34995711.
- ^ Adam SA (2001). "The nuclear pore complex". Genome Biology. 2 (9): reviews0007.1. doi:10.1186/gb-2001-2-9-reviews0007. PMC 138961. PMID 11574060.
- ^ Ibarra A, Hetzer MW (February 2015). "Nuclear pore proteins and the control of genome functions". Genes & Development. 29 (4): 337–349. doi:10.1101/gad.256495.114. PMC 4335290. PMID 25691464.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search