
Back أولانزابين Arabic الانزاپین AZB Olanzapina Catalan Olanzapine Welsh Olanzapin Danish Olanzapin German Ολανζαπίνη Greek Olanzapino Esperanto Olanzapina Spanish Olansapiin Estonian
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zyprexa, Zypine, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601213 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular injection |
| Drug class | Atypical antipsychotic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60–65%[5][6][7] |
| Protein binding | 93%[8] |
| Metabolism | Liver (direct glucuronidation and CYP1A2 mediated oxidation) |
| Elimination half-life | 33 hours, 51.8 hours (elderly)[8] |
| Excretion | Urine (57%; 7% as unchanged drug), faeces (30%)[8][9] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.125.320 |
| Chemical and physical data | |
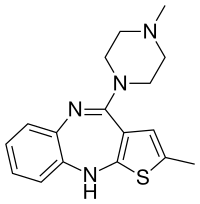
| Formula | C17H20N4S |
| Molar mass | 312.44 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 195 °C (383 °F) |
| Solubility in water | Practically insoluble in water mg/mL (20 °C) |
| |
| |
| | |
Olanzapine, sold under the brand name Zyprexa among others, is an atypical antipsychotic primarily used to treat schizophrenia and bipolar disorder.[10] For schizophrenia, it can be used for both new-onset disease and long-term maintenance.[10] It is taken by mouth or by injection into a muscle.[10]
Common side effects include weight gain, movement disorders, dizziness, feeling tired, constipation, and dry mouth.[10] Other side effects include low blood pressure with standing, allergic reactions, neuroleptic malignant syndrome, high blood sugar, seizures, and tardive dyskinesia.[10] In older people with dementia, its use increases the risk of death.[10] Use in the later part of pregnancy may result in a movement disorder in the baby for some time after birth.[10] Although how it works is not entirely clear, it blocks dopamine and serotonin receptors.[10]
Olanzapine was patented in 1991 and approved for medical use in the United States in 1996.[10][11] It is available as a generic medication.[10] In 2021, it was the 164th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[12][13] Eli Lilly also markets olanzapine in a fixed-dose combination with fluoxetine as olanzapine/fluoxetine (Symbyax).[14] It is on the World Health Organization's List of Essential Medicines.[15]
- ^ Cite error: The named reference
Drugnameswas invoked but never defined (see the help page). - ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Zyprexa EPAR". European Medicines Agency. 27 September 1996. Retrieved 27 February 2024.
- ^ Kassahun K, Mattiuz E, Nyhart E, Obermeyer B, Gillespie T, Murphy A, et al. (January 1997). "Disposition and biotransformation of the antipsychotic agent olanzapine in humans". Drug Metabolism and Disposition. 25 (1): 81–93. PMID 9010634.
- ^ Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM (September 1999). "Olanzapine. Pharmacokinetic and pharmacodynamic profile". Clinical Pharmacokinetics. 37 (3): 177–193. doi:10.2165/00003088-199937030-00001. PMID 10511917.
- ^ Mauri MC, Volonteri LS, Colasanti A, Fiorentini A, De Gaspari IF, Bareggi SR (2007). "Clinical pharmacokinetics of atypical antipsychotics: a critical review of the relationship between plasma concentrations and clinical response". Clinical Pharmacokinetics. 46 (5): 359–388. doi:10.2165/00003088-200746050-00001. PMID 17465637. S2CID 43859718.
- ^ a b c Cite error: The named reference
TGAwas invoked but never defined (see the help page). - ^ Cite error: The named reference
MSRwas invoked but never defined (see the help page). - ^ a b c d e f g h i j "Olanzapine, Olanzapine Pamoate Monograph for Professionals". Drugs.com. AHFS. Retrieved 24 December 2018.
- ^ Taylor D, Paton C, Kapur S (2015). The Maudsley Prescribing Guidelines in Psychiatry (12th ed.). London, U K: Wiley-Blackwell. p. 16. ISBN 978-1-118-75460-3.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Olanzapine – Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
- ^ "FDA Approves Symbyax as First Medication for Treatment-Resistant Depression". Lilly. Eli Lilly. Retrieved 17 March 2021.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search