
Back فسفرة تأكسدية Arabic Окислително фосфорилиране Bulgarian Oksidacijska fosforilacija BS Fosforilació oxidativa Catalan Oxidative Phosphorylierung German Οξειδωτική φωσφορυλίωση Greek Fosforilación oxidativa Spanish Oksüdatiivne fosforüülimine Estonian Fosforilazio oxidatibo Basque فسفرگیری اکسایشی Persian
Oxidative phosphorylation (UK /ɒkˈsɪd.ə.tɪv/, US /ˈɑːk.sɪˌdeɪ.tɪv/ [1]) or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobic glycolysis.
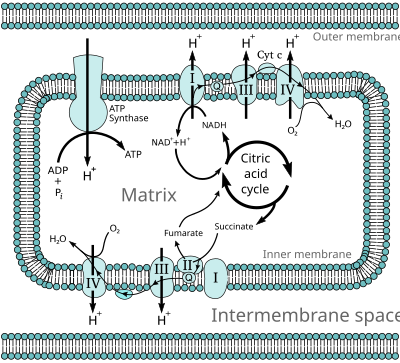
The energy stored in the chemical bonds of glucose is released by the cell in the citric acid cycle, producing carbon dioxide and the energetic electron donors NADH and FADH. Oxidative phosphorylation uses these molecules and O2 to produce ATP, which is used throughout the cell whenever energy is needed. During oxidative phosphorylation, electrons are transferred from the electron donors to a series of electron acceptors in a series of redox reactions ending in oxygen, whose reaction releases half of the total energy.[2]
In eukaryotes, these redox reactions are catalyzed by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cell's outer membrane. These linked sets of proteins are called the electron transport chain. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.
The energy transferred by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and the resulting electrical potential across this membrane. This store of energy is tapped when protons flow back across the membrane and down the potential energy gradient, through a large enzyme called ATP synthase in a process called chemiosmosis. The ATP synthase uses the energy to transform adenosine diphosphate (ADP) into adenosine triphosphate, in a phosphorylation reaction. The reaction is driven by the proton flow, which forces the rotation of a part of the enzyme. The ATP synthase is a rotary mechanical motor.
Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging and senescence. The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.
- ^ "oxidative Meaning in the Cambridge English Dictionary". dictionary.cambridge.org. Archived from the original on 24 January 2018. Retrieved 28 April 2018.
- ^ Voet, D.; Voet, J. G. (2004). "Biochemistry", 3rd ed., p. 804, Wiley.ISBN 0-471-19350-X.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search