
Back دورة الأكسجين Arabic Cicle de l'oxigen Catalan Koloběh kyslíku Czech Sauerstoffkreislauf German Κύκλος του οξυγόνου Greek Ciclo del oxígeno Spanish Hapnikuringe Estonian چرخه اکسیژن Persian Hapen kiertokulku Finnish Cycle de l'oxygène French
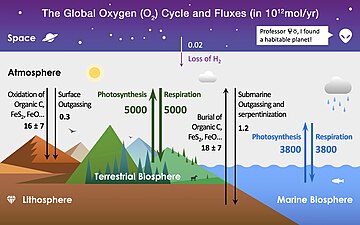
The major fluxes between these reservoirs are shown in colored arrows, where the green arrows are related to the terrestrial biosphere, blue arrows are related to the marine biosphere, black arrows are related to the lithosphere, and the purple arrow is related to space (not a reservoir, but also contributes to the atmospheric O2).[1]
The value of photosynthesis or net primary productivity (NPP) can be estimated through the variation in the abundance and isotopic composition of atmospheric O2.[2][3]
The rate of organic carbon burial was derived from estimated fluxes of volcanic and hydrothermal carbon.[4][5]
Oxygen cycle refers to the movement of oxygen through the atmosphere (air), biosphere (plants and animals) and the lithosphere (the Earth’s crust). The oxygen cycle demonstrates how free oxygen is made available in each of these regions, as well as how it is used. The oxygen cycle is the biogeochemical cycle of oxygen atoms between different oxidation states in ions, oxides, and molecules through redox reactions within and between the spheres/reservoirs of the planet Earth.[1] The word oxygen in the literature typically refers to the most common oxygen allotrope, elemental/diatomic oxygen (O2), as it is a common product or reactant of many biogeochemical redox reactions within the cycle.[2] Processes within the oxygen cycle are considered to be biological or geological and are evaluated as either a source (O2 production) or sink (O2 consumption).[1][2]
Oxygen is one of the most common elements on Earth and represents a large portion of each main reservoir. By far the largest reservoir of Earth's oxygen is within the silicate and oxide minerals of the crust and mantle (99.5% by weight).[6] The Earth's atmosphere, hydrosphere, and biosphere together hold less than 0.05% of the Earth's total mass of oxygen. Besides O2, additional oxygen atoms are present in various forms spread throughout the surface reservoirs in the molecules of biomass, H2O, CO2, HNO3, NO, NO2, CO, H2O2, O3, SO2, H2SO4, MgO, CaO, Al2O3, SiO2, and PO4.[7]
- ^ a b c Knoll AH, Canfield DE, Konhauser K (2012). "7". Fundamentals of geobiology. Chichester, West Sussex: John Wiley & Sons . pp. 93–104. ISBN 978-1-118-28087-4. OCLC 793103985.
- ^ a b c Petsch ST (2014). "The Global Oxygen Cycle". Treatise on Geochemistry. Elsevier. pp. 437–473. doi:10.1016/b978-0-08-095975-7.00811-1. ISBN 978-0-08-098300-4.
- ^ Keeling RF, Shertz SR (August 1992). "Seasonal and interannual variations in atmospheric oxygen and implications for the global carbon cycle". Nature. 358 (6389): 723–727. Bibcode:1992Natur.358..723K. doi:10.1038/358723a0. S2CID 4311084.
- ^ Holland HD (2002). "Volcanic gases, black smokers, and the great oxidation event". Geochimica et Cosmochimica Acta. 66 (21): 3811–3826. Bibcode:2002GeCoA..66.3811H. doi:10.1016/S0016-7037(02)00950-X.
- ^ Lasaga AC, Ohmoto H (2002). "The oxygen geochemical cycle: dynamics and stability". Geochimica et Cosmochimica Acta. 66 (3): 361–381. Bibcode:2002GeCoA..66..361L. doi:10.1016/S0016-7037(01)00685-8.
- ^ Falkowski PG, Godfrey LV (August 2008). "Electrons, life and the evolution of Earth's oxygen cycle". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 363 (1504): 2705–16. doi:10.1098/rstb.2008.0054. PMC 2606772. PMID 18487127.
- ^ Cite error: The named reference
:2was invoked but never defined (see the help page).
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search