
Back فينيل بروبانويد Arabic Фенилпропаноид Bulgarian Fenilpropanoide Catalan Fenylpropanoidy Czech Phenylpropanoide German Φαινυλοπροπανοειδή Greek Fenilpropanoides Spanish Phénylpropanoïde French Phenylpropanoid FRR Fenilpropanoide Italian


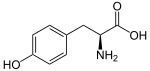
The phenylpropanoids are a diverse family of organic compounds that are biosynthesized by plants from the amino acids phenylalanine and tyrosine in the shikimic acid pathway.[1] Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids.[2] The coumaroyl component is produced from cinnamic acid.
Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and also mediate plant-pollinator interactions as floral pigments and scent compounds.
- ^ Barros J, Serrani-Yarce JC, Chen F, Baxter D, Venables BJ, Dixon RA (2016). "Role of bifunctional ammonia-lyase in grass cell wall biosynthesis". Nat. Plants. 2 (6): 16050. doi:10.1038/nplants.2016.50. PMID 27255834. S2CID 3462127.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vogt, T. (2010). "Phenylpropanoid Biosynthesis". Molecular Plant. 3: 2–20. doi:10.1093/mp/ssp106. PMID 20035037.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search