Back حمض الفسفوروز Arabic فوسفورو اسید AZB ফসফরাস অ্যাসিড Bengali/Bangla Fosforasta kiselina BS Àcid fosforós Catalan Kyselina fosforitá Czech Phosphonsäure German Ácido fosforoso Spanish فسفرو اسید Persian Fosforihapoke Finnish
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Phosphonic acid
| |||
| Systematic IUPAC name
Phosphorous acid | |||
| Other names
Dihydroxyphosphine oxide
Dihydroxy(oxo)-λ5-phosphane | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.033.682 | ||
| EC Number |
| ||
| 1619 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2834 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| H3PO3 | |||
| Molar mass | 81.99 g/mol | ||
| Appearance | white solid deliquescent | ||
| Density | 1.651 g/cm3 (21 °C) | ||
| Melting point | 73.6 °C (164.5 °F; 346.8 K) | ||
| Boiling point | 200 °C (392 °F; 473 K) (decomposes) | ||
| 310 g/100 mL | |||
| Solubility | soluble in ethanol | ||
| Acidity (pKa) | 1.1, 6.7 | ||
| −42.5·10−6 cm3/mol | |||
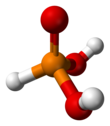
| Structure | |||
| pseudo-tetrahedral | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
skin irritant | ||
| GHS labelling:[1] | |||
 
| |||
| Danger | |||
| H302, H314 | |||
| P260, P264, P270, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P363, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | Sigma-Aldrich | ||
| Related compounds | |||
Related compounds
|
H3PO4 (i.e., PO(OH)3) H3PO2 (i.e., H2PO(OH)) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Phosphorous acid (or phosphonic acid) is the compound described by the formula H3PO3. This acid is diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.
- ^ "Phosphorous acid". pubchem.ncbi.nlm.nih.gov.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search