
Back ريلوزول Arabic ریلوزول AZB Rilwsol Welsh Riluzol German Ριλουζόλη Greek Riluzol Spanish Rilusool Estonian ریلوزول Persian Rilutsoli Finnish Riluzole French
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Rilutek, Tiglutik, Exservan, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696013 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60±18%[1] |
| Protein binding | 97%[1] |
| Metabolism | Hepatic (CYP1A2)[1] |
| Elimination half-life | 9–15 hours[1] |
| Excretion | Urine (90%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.124.754 |
| Chemical and physical data | |
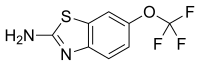
| Formula | C8H5F3N2OS |
| Molar mass | 234.20 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Riluzole is a medication used to treat amyotrophic lateral sclerosis and other motor neuron diseases. Riluzole delays the onset of ventilator-dependence or tracheostomy in some people and may increase survival by two to three months.[2] Riluzole is available in tablet and liquid form.
- ^ a b c d e "PRODUCT INFORMATION RILUTEK® (riluzole) Tablets" (PDF). TGA eBusiness Services. sanofi-aventis australia pty ltd. January 6, 2009. Retrieved February 18, 2014.
- ^ Miller RG, Mitchell JD, Moore DH (March 2012). "Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND)". The Cochrane Database of Systematic Reviews. 2012 (3): CD001447. doi:10.1002/14651858.CD001447.pub3. PMC 7055506. PMID 22419278.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search