
Back سكارين Arabic ساخارین AZB Сахарын Byelorussian Цукрын BE-X-OLD Захарин Bulgarian স্যাকারিন Bengali/Bangla Sacarina Catalan Sacharin Czech Sakkarin Danish Saccharin German
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
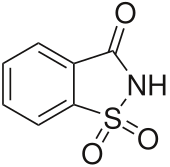
1H-1λ6,2-Benzothiazole-1,1,3(2H)-trione | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.202 |
| E number | E954 (glazing agents, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H5NO3S | |
| Molar mass | 183.18 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 0.828 g/cm3 |
| Melting point | 228.8 to 229.7 °C (443.8 to 445.5 °F; 501.9 to 502.8 K) |
| 1 g per 290 mL | |
| Acidity (pKa) | 1.6[4] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Saccharin, also called saccharine, benzosulfimide, or E954, or used in saccharin sodium or saccharin calcium forms, is a non-nutritive artificial sweetener.[1][5] Saccharin is a sultam that is about 500 times sweeter than sucrose, but has a bitter or metallic aftertaste, especially at high concentrations.[1] It is used to sweeten products, such as drinks, candies, baked goods, tobacco products, excipients, and for masking the bitter taste of some medicines.[1][5] It appears as white crystals and is odorless.[1]
- ^ a b c d e "Saccharin". PubChem, US National Library of Medicine. 13 June 2023. Retrieved 15 June 2023.
- ^ "Saccharin (CAS: 81-07-2)". Merck Millipore. 2023. Retrieved August 22, 2022.
- ^ NCERT Chemistry Part II Textbook for Class XII. Delhi: NCERT. 2021. p. 449. ISBN 81-7450-716-7.
- ^ Cite error: The named reference
BellHigginsonwas invoked but never defined (see the help page). - ^ a b "Saccharin". Drugs.com. 16 August 2022. Retrieved 15 June 2023.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search