
Back ساكوبيتريل / فالسارتان Arabic Sacubitril/valsartan French サクビトリル・バルサルタン Japanese Сакубитрил/валсартан Macedonian ସାକୁବିଟ୍ରିଲ/ଭାଲ୍ସାର୍ଟାନ OR LCZ696 Serbo-Croatian LCZ696 Serbian Sacubitril/valsartan Vietnamese
 | |
| Combination of | |
|---|---|
| Sacubitril | Neprilysin inhibitor |
| Valsartan | Angiotensin II receptor antagonist |
| Clinical data | |
| Trade names | Entresto, Azmarda, Neparvis, others |
| Other names | LCZ696 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a615039 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
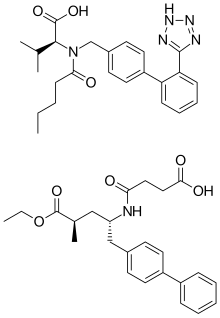
| Formula | C96H120N12Na6O21 |
| Molar mass | 1916.018 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Sacubitril/valsartan, sold under the brand name Entresto, is a fixed-dose combination medication for use in heart failure. It consists of the neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan. The combination is sometimes described as an "angiotensin receptor-neprilysin inhibitor" (ARNi).[9] In 2016, the American College of Cardiology/American Heart Association Task Force recommended it as a replacement for an ACE inhibitor or an angiotensin receptor blocker in people with heart failure with reduced ejection fraction.[10]
Potential side effects include angioedema, nephrotoxicity, and low blood pressure.[10]
It was approved for medical use in the United States and in the European Union in 2015,[11][12][13][7] and in Australia in 2016.[1] In 2021, it was the 189th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[14][15] It is available as a generic medication.[16]
- ^ a b c "Entresto 24/26 tablets, Entresto 49/51 tablets, Entresto 97/103 tablets (sacubitril/valsartan) Product Information". Therapeutic Goods Administration (TGA). Novartis. Archived from the original on 11 November 2020. Retrieved 21 September 2020.
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2016". Therapeutic Goods Administration (TGA). 21 June 2022. Archived from the original on 10 April 2023. Retrieved 10 April 2023.
- ^ AusPAR for sacubitril / valsartan salt complex (PDF) (Report). Therapeutic Goods Administration (TGA). September 2016.
- ^ "Health Canada New Drug Authorizations: 2015 Highlights". Health Canada. 4 May 2016. Retrieved 7 April 2024.
- ^ "Entresto 24 mg/26 mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). Archived from the original on 24 January 2021. Retrieved 21 September 2020.
- ^ Cite error: The named reference
Entresto FDA labelwas invoked but never defined (see the help page). - ^ a b "Entresto EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 27 February 2021. Retrieved 21 September 2020.
- ^ "Neparvis EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 28 December 2020. Retrieved 23 September 2020.
- ^ Hubers SA, Brown NJ (March 2016). "Combined Angiotensin Receptor Antagonism and Neprilysin Inhibition". Circulation. 133 (11): 1115–1124. doi:10.1161/CIRCULATIONAHA.115.018622. PMC 4800749. PMID 26976916.
- ^ a b Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. (September 2016). "2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America". Circulation. 134 (13): e282–e293. doi:10.1161/CIR.0000000000000435. PMID 27208050.
- ^ "FDA approves new drug to treat heart failure" (Press release). U.S. Food and Drug Administration (FDA). 7 July 2015. Archived from the original on 26 January 2018.
- ^ "Entresto (sacubitril/valsartan) Tablets". U.S. Food and Drug Administration (FDA). 14 August 2015. Archived from the original on 31 March 2021. Retrieved 22 September 2020.
- ^ Thompson A (12 June 2015). Summary review of LCZ696, a fixed-dose combination of valsartan and sacubitril (PDF) (Report). Center for Drug Evaluation and Research. 207620Orig1s000. Partially redacted.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Sacubitril; Valsartan - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
- ^ "FDA Roundup: May 31, 2024". U.S. Food and Drug Administration (FDA) (Press release). 31 May 2024. Retrieved 31 May 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search