
Back سيليجيلين Arabic سلژیلین AZB Selegilin Welsh Selegilin German Selegilina Spanish سلژیلین Persian Selegiliini Finnish Sélégiline French סלג'ילין HE Szelegilin Hungarian
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /səˈlɛdʒɪliːn/ sə-LEJ-i-leen ("seh-LEH-ji-leen")[1][2] |
| Trade names | Eldepryl, Jumex, Zelapar, Emsam, Anipryl, others[3] |
| Other names | L-Deprenyl; L-Deprenil; L-Deprenalin; L-Deprenaline; L-E-250; L-Phenylisopropyl |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697046 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | • Oral (tablet, capsule)[4][5] • Buccal (ODT)[6][7] • Transdermal (patch)[8][9] |
| Drug class | Monoamine oxidase inhibitor; Catecholaminergic activity enhancer; Norepinephrine releasing agent; Antiparkinsonian; Antidepressant; Neuroprotective |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: 4–10%[5][11][12] ODT: ~5–8× oral[13][7][14] Patch: 75%[9] |
| Protein binding | 85–90%[9][8][6] |
| Metabolism | Liver, other tissues (CYP2B6, CYP2C19, others)[5][18][9][19] |
| Metabolites | • Desmethylselegiline (DMS) • Levomethamphetamine (L-MA) • Levoamphetamine (L-A) |
| Elimination half-life | Oral: • S (single): 1.2–3.5 h[5] • S (multi): 7.7–9.7 h[5][12] • DMS (single): 2.2–3.8 h[5] • DMS (multi): 9.5 h[5] • L-MA: 14–21 h[5][7] • L-A: 16–18 h[5][7] ODT: • S (single): 1.3 h[6] • S (multi): 10 h[6] Patch: • S: 20 h[12][8] |
| Excretion | Urine (87%):[15][16][7][5][17] • L-MA: 20–63% • L-A: 9–26% • DMS: 1% • S: 0.01–0.03% Feces: 15%[15][7] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.109.269 |
| Chemical and physical data | |
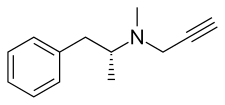
| Formula | C13H17N |
| Molar mass | 187.286 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Levorotatory enantiomer |
| |
| |
| (verify) | |
Selegiline, also known as L-deprenyl and sold under the brand names Eldepryl, Zelapar, and Emsam among others, is a medication which is used in the treatment of Parkinson's disease and major depressive disorder.[4][6][8][3] It has also been studied for a variety of other indications, but has not been formally approved for any other use.[20][21] The medication in the form licensed for depression has modest effectiveness for this condition that is similar to that of other antidepressants.[21][22][23] Selegiline is provided as a swallowed tablet or capsule[4][5] or an orally disintegrating tablet (ODT)[6][7] for Parkinson's disease and as a patch applied to skin for depression.[8][9]
Side effects of selegiline occurring more often than with placebo include insomnia, dry mouth, dizziness, nervousness, abnormal dreams, and application site reactions (with the patch form), among others.[21][22][24][4][8] At high doses, selegiline has the potential for dangerous food and drug interactions, such as the tyramine-related "cheese reaction" or hypertensive crisis and risk of serotonin syndrome.[9][25][5] However, doses within the approved clinical range appear to have little to no risk of these interactions.[9][25][5] In addition, the ODT and transdermal patch forms of selegiline have reduced risks of such interactions compared to the conventional oral form.[7][9] Selegiline has no known misuse potential or dependence liability and is not a controlled substance.[26][27][28][29][8]
Selegiline acts as a monoamine oxidase inhibitor (MAOI) and thereby increases levels of monoamine neurotransmitters in the brain.[30][11][25][5] At typical clinical doses used for Parkinson's disease, selegiline is a selective and irreversible inhibitor of monoamine oxidase B (MAO-B), increasing brain levels of dopamine.[30][11][25][5] At higher doses, it loses its specificity for MAO-B and also inhibits monoamine oxidase A (MAO-A), which increases serotonin and norepinephrine levels in the brain as well.[30][11][25][5] In addition to its MAOI activity, selegiline is a catecholaminergic activity enhancer (CAE) and enhances the impulse-mediated release of norepinephrine and dopamine in the brain.[31][32][33][34][25] This action may be mediated by TAAR1 agonism.[35][36][37] After administration, selegiline partially metabolizes into levomethamphetamine and levoamphetamine, which act as norepinephrine releasing agents (NRAs) and may contribute to its therapeutic and adverse effects.[38][28][39] The levels of these metabolites are much lower with the ODT and transdermal patch forms of selegiline.[7][9] Chemically, selegiline is a substituted amphetamine,[40] a derivative of methamphetamine,[40] and the purified levorotatory enantiomer of deprenyl (the racemic form).[41][20]
Deprenyl was discovered and studied in the early 1960s.[41][20] Subsequently, selegiline was purified from deprenyl and was studied and developed itself.[41] Selegiline was first introduced for medical use in Hungary in 1977.[42] It was subsequently approved in the United Kingdom in 1982 and in the United States in 1989.[42][43] The ODT was approved in the United States in 2006 and in the European Union in 2010, while the patch was introduced in the United States in 2006.[42][20] Selegiline was the first selective MAO-B inhibitor to be discovered and marketed.[13][44][45] In addition to its medical use, there has been interest in selegiline as a potential anti-aging drug and nootropic.[46][47][48] However, effects of this sort are controversial and uncertain.[46][49][50][51] Generic versions of selegiline are available in the case of the conventional oral form but not in the case of the ODT or transdermal patch forms.[52][53]
- ^ Cite error: The named reference
Parkinsons.org2018was invoked but never defined (see the help page). - ^ Cite error: The named reference
Acosta2020was invoked but never defined (see the help page). - ^ a b "Selegiline". Drugs.com. Archived from the original on July 3, 2024. Retrieved February 7, 2016.
- ^ a b c d Cite error: The named reference
PillLabelwas invoked but never defined (see the help page). - ^ a b c d e f g h i j k l m n o p Mahmood I (August 1997). "Clinical pharmacokinetics and pharmacodynamics of selegiline. An update". Clin Pharmacokinet. 33 (2): 91–102. doi:10.2165/00003088-199733020-00002. PMID 9260033.
- ^ a b c d e f Cite error: The named reference
ODTLabelwas invoked but never defined (see the help page). - ^ a b c d e f g h i Poston KL, Waters C (October 2007). "Zydis selegiline in the management of Parkinson's disease". Expert Opin Pharmacother. 8 (15): 2615–2624. doi:10.1517/14656566.8.15.2615. PMID 17931095.
- ^ a b c d e f g "EMSAM® (Selegiline Transdermal System) Label" (PDF). Food and Drug Administration. July 2017. Retrieved July 2, 2024.
- ^ a b c d e f g h i Cite error: The named reference
LeeChen2007was invoked but never defined (see the help page). - ^ Anvisa (March 31, 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published April 4, 2023). Archived from the original on August 3, 2023. Retrieved August 16, 2023.
- ^ a b c d Magyar K (2011). "The Pharmacology of Selegiline". In Youdim M, Riederer P (eds.). Monoamine Oxidases and Their Inhibitors. International Review of Neurobiology. Vol. 100. Academic Press. pp. 65–84. doi:10.1016/B978-0-12-386467-3.00004-2. ISBN 978-0-12-386467-3. PMID 21971003.
- ^ a b c Pae CU, Lim HK, Han C, Neena A, Lee C, Patkar AA (August 2007). "Selegiline transdermal system: current awareness and promise". Prog Neuropsychopharmacol Biol Psychiatry. 31 (6): 1153–1163. doi:10.1016/j.pnpbp.2007.04.020. PMID 17614182.
- ^ a b Löhle M, Storch A (November 2008). "Orally disintegrating selegiline for the treatment of Parkinson's disease". Expert Opin Pharmacother. 9 (16): 2881–2891. doi:10.1517/14656566.9.16.2881. PMID 18937619.
- ^ Clarke A, Brewer F, Johnson ES, Mallard N, Hartig F, Taylor S, et al. (November 2003). "A new formulation of selegiline: improved bioavailability and selectivity for MAO-B inhibition". Journal of Neural Transmission. 110 (11): 1241–1255. doi:10.1007/s00702-003-0036-4. PMID 14628189. S2CID 711419.
- ^ a b Heinonen EH, Anttila MI, Lammintausta RA (December 1994). "Pharmacokinetic aspects of l-deprenyl (selegiline) and its metabolites". Clin Pharmacol Ther. 56 (6 Pt 2): 742–749. doi:10.1038/clpt.1994.204. PMID 7995016.
- ^ Heinonen EH, Myllylä V, Sotaniemi K, Lamintausta R, Salonen JS, Anttila M, et al. (November 1989). "Pharmacokinetics and metabolism of selegiline". Acta Neurologica Scandinavica. Supplementum. 126: 93–99. doi:10.1111/j.1600-0404.1989.tb01788.x. PMID 2515726. S2CID 221440315.
- ^ Chrisp P, Mammen GJ, Sorkin EM (May 1991). "Selegiline: A Review of its Pharmacology, Symptomatic Benefits and Protective Potential in Parkinson's Disease". Drugs Aging. 1 (3): 228–248. doi:10.2165/00002512-199101030-00006. PMID 1794016.
- ^ Rodrigues AD (June 2022). "Drug Interactions Involving 17α-Ethinylestradiol: Considerations Beyond Cytochrome P450 3A Induction and Inhibition". Clin Pharmacol Ther. 111 (6): 1212–1221. doi:10.1002/cpt.2383. PMID 34342002.
- ^ Hidestrand M, Oscarson M, Salonen JS, Nyman L, Pelkonen O, Turpeinen M, et al. (November 2001). "CYP2B6 and CYP2C19 as the major enzymes responsible for the metabolism of selegiline, a drug used in the treatment of Parkinson's disease, as revealed from experiments with recombinant enzymes". Drug Metab Dispos. 29 (11): 1480–1484. PMID 11602525.
- ^ a b c d Cite error: The named reference
Miklya2016was invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
RossanoCaiazzaSobrino2023was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
CitromeGoldbergPortland2013was invoked but never defined (see the help page). - ^ Cite error: The named reference
CiprianiFurukawaSalanti2018was invoked but never defined (see the help page). - ^ Cite error: The named reference
RobinsonAmsterdam2008was invoked but never defined (see the help page). - ^ a b c d e f Gerlach M, Youdim MB, Riederer P (December 1996). "Pharmacology of selegiline". Neurology. 47 (6 Suppl 3): S137–S145. doi:10.1212/wnl.47.6_suppl_3.137s. PMID 8959982.
- ^ Cite error: The named reference
FinbergRabey2016was invoked but never defined (see the help page). - ^ Cite error: The named reference
FabbriniAbbruzzeseMarconi2012was invoked but never defined (see the help page). - ^ a b Yasar S, Goldberg JP, Goldberg SR (January 1, 1996). "Are metabolites of l-deprenyl (Selegiline) useful or harmful? Indications from preclinical research". Deprenyl — Past and Future. Journal of Neural Transmission. Supplementum. Vol. 48. pp. 61–73. doi:10.1007/978-3-7091-7494-4_6. ISBN 978-3-211-82891-5. PMID 8988462.
- ^ Cite error: The named reference
NickelSzelenyiSchulze1994was invoked but never defined (see the help page). - ^ a b c Heinonen EH, Lammintausta R (1991). "A review of the pharmacology of selegiline". Acta Neurologica Scandinavica. Supplementum. 136: 44–59. doi:10.1111/j.1600-0404.1991.tb05020.x. PMID 1686954.
- ^ Knoll J (1997). "Istoriia deprenil--pervogo selektivnogo ingibitora monoaminoksidazy tipa B" [History of deprenyl--the first selective inhibitor of monoamine oxidase type B]. Voprosy Meditsinskoi Khimii. 43 (6): 482–493. PMID 9503565.
- ^ Knoll J (February 1998). "(-)Deprenyl (selegiline), a catecholaminergic activity enhancer (CAE) substance acting in the brain". Pharmacol Toxicol. 82 (2): 57–66. doi:10.1111/j.1600-0773.1998.tb01399.x. PMID 9498233.
- ^ Cite error: The named reference
Miklya2014awas invoked but never defined (see the help page). - ^ Cite error: The named reference
GasznerMiklya2006was invoked but never defined (see the help page). - ^ Harsing LG, Timar J, Miklya I (August 2023). "Striking Neurochemical and Behavioral Differences in the Mode of Action of Selegiline and Rasagiline". Int J Mol Sci. 24 (17): 13334. doi:10.3390/ijms241713334. PMC 10487936. PMID 37686140.
- ^ Shimazu S, Miklya I (May 2004). "Pharmacological studies with endogenous enhancer substances: β-phenylethylamine, tryptamine, and their synthetic derivatives". Prog Neuropsychopharmacol Biol Psychiatry. 28 (3): 421–427. doi:10.1016/j.pnpbp.2003.11.016. PMID 15093948.
- ^ Berry MD (January 2007). "The potential of trace amines and their receptors for treating neurological and psychiatric diseases". Rev Recent Clin Trials. 2 (1): 3–19. doi:10.2174/157488707779318107. PMID 18473983.
- ^ Gerlach M, Reichmann H, Riederer P (2012). "A critical review of evidence for preclinical differences between rasagiline and selegiline". Basal Ganglia. 2 (4): S9–S15. doi:10.1016/j.baga.2012.04.032.
- ^ Rothman RB, Baumann MH (October 2003). "Monoamine transporters and psychostimulant drugs". Eur J Pharmacol. 479 (1–3): 23–40. doi:10.1016/j.ejphar.2003.08.054. PMID 14612135.
- ^ a b Kraemer T, Maurer HH (April 2002). "Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives". Ther Drug Monit. 24 (2): 277–289. doi:10.1097/00007691-200204000-00009. PMID 11897973.
- ^ a b c Parnham MJ (1993). "The History of l-Deprenyl". Inhibitors of Monoamine Oxidase B: Pharmacology and Clinical Use in Neurodegenerative Disorders. Milestones in Drug Therapy. Basel: Birkhäuser Basel. pp. 237–251. doi:10.1007/978-3-0348-6348-3_12. ISBN 978-3-0348-6349-0.
- ^ a b c Tábi T, Vécsei L, Youdim MB, Riederer P, Szökő É (May 2020). "Selegiline: a molecule with innovative potential". J Neural Transm (Vienna). 127 (5): 831–842. doi:10.1007/s00702-019-02082-0. PMC 7242272. PMID 31562557.
- ^ Cite error: The named reference
MylanHist2011was invoked but never defined (see the help page). - ^ Cite error: The named reference
HoffmanOlsonSchoffstall2023was invoked but never defined (see the help page). - ^ Cite error: The named reference
Golbe1988was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
SchifanoCatalaniSharif2022was invoked but never defined (see the help page). - ^ Knoll J (2001). "Antiaging compounds: (-)deprenyl (selegeline) and (-)1-(benzofuran-2-yl)-2-propylaminopentane, [(-)BPAP], a selective highly potent enhancer of the impulse propagation mediated release of catecholamine and serotonin in the brain". CNS Drug Rev. 7 (3): 317–345. doi:10.1111/j.1527-3458.2001.tb00202.x. PMC 6494119. PMID 11607046.
- ^ Schneider LS, Tariot PN, Goldstein B (December 1994). "Therapy with l-deprenyl (selegiline) and relation to abuse liability". Clin Pharmacol Ther. 56 (6 Pt 2): 750–756. doi:10.1038/clpt.1994.205. PMID 7995017.
- ^ Blazer DG, Yaffe K, Liverman CT (July 21, 2015). Risk and Protective Factors and Interventions: General Cognitive Aging Interventions and Next Steps. National Academies Press (US). Retrieved July 5, 2024.
- ^ Brown RP, Gerbarg PL (2008). Muskin PR (ed.). "Integrative Psychopharmacology: A Practical Approach to Herbs and Nutrients in Psychiatry". Review of Psychiatry. Complementary and Alternative Medicine and Psychiatry. 19 (1). American Psychiatric Publishing: 1–66 (39). ISBN 978-1-58562-827-8. Retrieved July 5, 2024.
- ^ Finberg JP (April 2019). "Inhibitors of MAO-B and COMT: their effects on brain dopamine levels and uses in Parkinson's disease". Journal of Neural Transmission. 126 (4): 433–448. doi:10.1007/s00702-018-1952-7. PMID 30386930.
- ^ Cite error: The named reference
Drugs@FDAwas invoked but never defined (see the help page). - ^ Cite error: The named reference
AsnisHenderson2014was invoked but never defined (see the help page).
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search