
Back Serin/treonin-specifična protein-kinaza BS Serin/threonin kináza Czech Serina/treonina proteína cinasa Spanish پروتئین کیناز اختصاصی سرین/ترئونین Persian Sérine/thréonine protéine kinase French Serina/treonina proteína quinase Galician Serina/treonina protein kinase ID Serin/treonin-specifična proteinska kinaza Serbo-Croatian Serin/treonin-specifična proteinska kinaza Serbian سیرین تھریونین حرکی خامرہ Urdu
| Protein-serine/threonine kinases | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC no. | 2.7.11.- | ||||||||
| CAS no. | 9026-43-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||

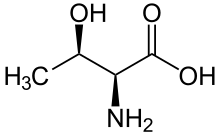
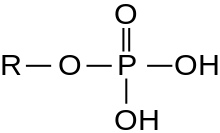

A serine/threonine protein kinase (EC 2.7.11.-) is a kinase enzyme, in particular a protein kinase, that phosphorylates the OH group of the amino-acid residues serine or threonine, which have similar side chains. At least 350 of the 500+ human protein kinases are serine/threonine kinases (STK).[2]
In enzymology, the term serine/threonine protein kinase describes a class of enzymes in the family of transferases, that transfer phosphates to the oxygen atom of a serine or threonine side chain in proteins. This process is called phosphorylation. Protein phosphorylation in particular plays a significant role in a wide range of cellular processes and is a very important post-translational modification.[3][4][5][6][7][8][9]
The chemical reaction performed by these enzymes can be written as
- ATP + a protein ADP + a phosphoprotein
Thus, the two substrates of this enzyme are ATP and a protein, whereas its two products are ADP and phosphoprotein.
The systematic name of this enzyme class is ATP:protein phosphotransferase (non-specific).
- ^ Nowakowski, J.; Cronin, C. N.; McRee, D. E.; Knuth, M. W.; Nelson, C. G.; Pavletich, N. P.; Rogers, J.; Sang, B. C.; Scheibe, D. N.; Swanson, R. V.; Thompson, D. A. (2002). "Structures of the cancer-related Aurora-A, FAK, and EphA2 protein kinases from nanovolume crystallography". Structure. 10 (12): 1659–1667. doi:10.1016/S0969-2126(02)00907-3. PMID 12467573.
- ^ Modi, V; Dunbrack, RL (24 December 2019). "A Structurally-Validated Multiple Sequence Alignment of 497 Human Protein Kinase Domains". Scientific Reports. 9 (1): 19790. Bibcode:2019NatSR...919790M. doi:10.1038/s41598-019-56499-4. PMC 6930252. PMID 31875044.
- ^ Damuni Z, Reed LJ (1988). "Purification and properties of a protamine kinase and a type II casein kinase from bovine kidney mitochondria". Arch. Biochem. Biophys. 262 (2): 574–84. doi:10.1016/0003-9861(88)90408-0. PMID 2835010.
- ^ Baggio B, Pinna LA, Moret V, Siliprandi N (1970). "A simple procedure for the purification of rat liver phosvitin kinase". Biochim. Biophys. Acta. 212 (3): 515–7. doi:10.1016/0005-2744(70)90261-5. PMID 5456997.
- ^ Jergil B, Dixon GH (1970). "Protamine kinase from rainbow trout testis. Partial purification and characterization". J. Biol. Chem. 245 (2): 425–34. doi:10.1016/S0021-9258(18)63408-8. PMID 4312674.
- ^ Langan TA (1969). "Action of adenosine 3',5'-monophosphate-dependent histone kinase in vivo". J. Biol. Chem. 244 (20): 5763–5. doi:10.1016/S0021-9258(18)63626-9. PMID 4310608.
- ^ Takeuchi M, Yanagida M (1993). "A mitotic role for a novel fission yeast protein kinase dsk1 with cell cycle stage dependent phosphorylation and localization". Mol. Biol. Cell. 4 (3): 247–60. doi:10.1091/mbc.4.3.247. PMC 300923. PMID 8485317.
- ^ NF; Lützelberger, M; Weigmann, H; Klingenhoff, A; Shenoy, S; Käufer, NF (1997). "Functional analysis of the fission yeast Prp4 protein kinase involved in pre-mRNA splicing and isolation of a putative mammalian homologue". Nucleic Acids Res. 25 (5): 1028–35. doi:10.1093/nar/25.5.1028. PMC 146536. PMID 9102632.
- ^ Wang Y, Hofmann TG, Runkel L, Haaf T, Schaller H, Debatin K, Hug H (2001). "Isolation and characterization of cDNAs for the protein kinase HIPK2". Biochim. Biophys. Acta. 1518 (1–2): 168–72. doi:10.1016/S0167-4781(00)00308-0. PMID 11267674.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
