
Back نتريد الصوديوم Arabic Натрий нитри(д)чĕ CV Natriumnitrid German Natria nitrido Esperanto سدیم نیترید Persian Nátrium-nitrid Hungarian Natrium nitrida ID Nitruro di sodio Italian 窒化ナトリウム Japanese Natrium nitrida Malay
| |
| Names | |
|---|---|
| IUPAC name
Sodium nitride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.032.017 |
| EC Number |
|
| |
| |
| Properties | |
| Na3N | |
| Molar mass | 82.976 g/mol |
| Appearance | reddish brown or dark blue solid |
| Melting point | 87 °C (189 °F; 360 K)[1] (decomposes) |
| reacts | |
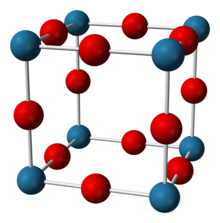
| Structure | |
| Cubic, cP4[2] | |
| Pm3m[2] | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-151 J/mol[2] |
| Related compounds | |
Other anions
|
Sodium amide Sodium imide |
Other cations
|
Lithium nitride Potassium nitride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium nitride is the inorganic compound with the chemical formula Na3N. In contrast to lithium nitride and some other nitrides, sodium nitride is an extremely unstable alkali metal nitride. It can be generated by combining atomic beams of sodium and nitrogen deposited onto a low-temperature sapphire substrate.[1] It readily decomposes into its elements:
- ^ a b Fischer, D., Jansen, M. (2002). "Synthesis and structure of Na3N". Angew Chem. 41 (10): 1755–1756. doi:10.1002/1521-3773(20020517)41:10<1755::AID-ANIE1755>3.0.CO;2-C. PMID 19750706.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Fischer, D.; Cancarevic, Z.; Schön, J. C.; Jansen, M. Z. (2004). "Synthesis and structure of K3N". Z. Anorg. Allg. Chem. 630 (1): 156. doi:10.1002/zaac.200300280.. 'Elusive Binary Compound Prepared' Chemical & Engineering News 80 No. 20 (20 May 2002) - ^ a b c Cite error: The named reference
Sangsterwas invoked but never defined (see the help page).
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
