
Back Sorbitol Afrikaans سوربيتول Arabic سوربیتول AZB Сарбіт Byelorussian Сорбитол Bulgarian সরবিটল Bengali/Bangla Sorbitol Catalan Sorbitol Czech Sorbitol Welsh Sorbitol Danish
| |

| |
| Names | |
|---|---|
| IUPAC name
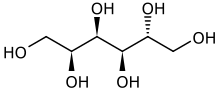
D-Glucitol[1]
| |
| Systematic IUPAC name
(2S,3R,4R,5R)-Hexane-1,2,3,4,5,6-hexol | |
| Other names
D-Sorbitol; Sorbogem; Sorbo
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.056 |
| E number | E420 (thickeners, ...) |
| KEGG | |
| MeSH | Sorbitol |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C6H14O6 | |
| Molar mass | 182.17 g/mol |
| Appearance | White crystalline powder |
| Density | 1.49 g/cm3[2] |
| Melting point | 94–96 °C (201–205 °F; 367–369 K)[2] |
| 2350 g/L[2] | |
| log P | -4.67[3] |
| -107.80·10−6 cm3/mol | |
| Pharmacology | |
| A06AD18 (WHO) A06AG07 (WHO) B05CX02 (WHO) V04CC01 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | > 100 °C (212 °F; 373 K)[2] |
| 420 °C (788 °F; 693 K)[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sorbitol (/ˈsɔː(r)bɪtɒl/), less commonly known as glucitol (/ˈɡluːsɪtɒl/), is a sugar alcohol with a sweet taste which the human body metabolizes slowly. It can be obtained by reduction of glucose, which changes the converted aldehyde group (−CHO) to a primary alcohol group (−CH2OH). Most sorbitol is made from potato starch, but it is also found in nature, for example in apples, pears, peaches, and prunes.[4] It is converted to fructose by sorbitol-6-phosphate 2-dehydrogenase. Sorbitol is an isomer of mannitol, another sugar alcohol; the two differ only in the orientation of the hydroxyl group on carbon 2.[5] While similar, the two sugar alcohols have very different sources in nature, melting points, and uses.
As an over-the-counter drug, sorbitol is used as a laxative to treat constipation.[6]
- ^ publications.iupac.org/pac/1996/pdf/6810x1919.pdf
- ^ a b c d e Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ "Sorbitol_msds".
- ^ Teo G, Suzuki Y, Uratsu SL, Lampinen B, Ormonde N, Hu WK, Dejong TM, Dandekar AM (2006). "Silencing leaf sorbitol synthesis alters long-distance partitioning and apple fruit quality". Proceedings of the National Academy of Sciences of the United States of America. 103 (49): 18842–7. Bibcode:2006PNAS..10318842T. doi:10.1073/pnas.0605873103. PMC 1693749. PMID 17132742.
- ^ Kearsley, M. W.; Deis, R. C. Sorbitol and Mannitol. In Sweeteners and Sugar Alternatives in Food Technology; Ames: Oxford, 2006; pp 249-249-261.
- ^ "Sorbitol". Drugs.com. 23 November 2021. Retrieved 8 July 2022.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
