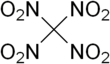Back تترانیترومتان AZB Tetranitromethan German Tetranitrometano Spanish تترانیترومتان Persian Tetranitrometaani Finnish Tétranitrométhane French Tetranitrometano Italian テトラニトロメタン Japanese Tetranitromethaan Dutch Tetranitrometan Polish
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetranitromethane
| |||
| Other names
TNM
Tetan | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.359 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1510 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
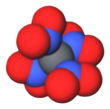
| C(NO2)4 | |||
| Molar mass | 196.04 g/mol | ||
| Appearance | Colorless to pale-yellow liquid or solid | ||
| Odor | Pungent | ||
| Density | 1.623 g/cm3 | ||
| Melting point | 13.8 °C (56.8 °F; 286.9 K) | ||
| Boiling point | 126 °C (259 °F; 399 K) | ||
| insoluble | |||
| Vapor pressure | 8 mmHg (20°C)[2] | ||
| -43.02·10−6 cm3/mol | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Oxidant, can form explosive mixtures | ||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H271, H301, H315, H319, H330, H335, H351 | |||
| P201, P202, P210, P220, P221, P260, P261, P264, P270, P271, P280, P281, P283, P284, P301+P310, P302+P352, P304+P340, P305+P351+P338, P306+P360, P308+P313, P310, P312, P320, P321, P330, P332+P313, P337+P313, P362, P370+P378, P371+P380+P375, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
18 ppm (rat, 4 hr) 100 ppm (cat, 20 min) 54 ppm (mouse, 4 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1 ppm (8 mg/m3)[2] | ||
REL (Recommended)
|
TWA 1 ppm (8 mg/m3)[2] | ||
IDLH (Immediate danger)
|
4 ppm[2] | ||
| Safety data sheet (SDS) | ICSC 1468 | ||
| Related compounds | |||
Related compounds
|
Hexanitroethane Octanitropentane Trinitromethane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tetranitromethane or TNM is an organic oxidizer with chemical formula C(NO2)4. Its chemical structure consists of four nitro groups attached to one carbon atom. In 1857 it was first synthesised by the reaction of sodium cyanoacetamide with nitric acid.[4]
- ^ Merck Index, 11th Edition, 9164.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0605". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Tetranitromethane". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ L. N. Shishkov (1857). "Sur la constitution de l'acetic fulminique et un nouvelle serie de corps derives de l'acide acetique". Annales de chimie et de physique. 49 (11): 310.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search