
Back يوديد التيتانيوم الرباعي Arabic تیتانیوم تترایودیود AZB Jodid titaničitý Czech Titan(IV)-iodid German تیتانیم تترایدید Persian Titaani(IV)jodidi Finnish Tétraiodure de titane French टाइटेनियम टेट्राआयोडाइड Hindi Tetraioduro di titanio Italian Иодид титана(IV) Russian
| |

| |
| Names | |
|---|---|
| IUPAC name
Titanium(IV) iodide
| |
| Other names
Titanium tetraiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.028.868 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| TiI4 | |
| Molar mass | 555.485 g/mol |
| Appearance | red-brown crystals |
| Density | 4.3 g/cm3 |
| Melting point | 150 °C (302 °F; 423 K) |
| Boiling point | 377 °C (711 °F; 650 K) |
| hydrolysis | |
| Solubility in other solvents | soluble in CH2Cl2 CHCl3 CS2 |
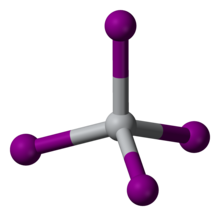
| Structure | |
| cubic (a = 12.21 Å) | |
| tetrahedral | |
| 0 D | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
violent hydrolysis corrosive |
| GHS labelling:[1] | |

| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
| Related compounds | |
Other anions
|
Titanium(IV) bromide Titanium(IV) chloride Titanium(IV) fluoride |
Other cations
|
Silicon tetraiodide Zirconium(IV) iodide Hafnium(IV) iodide |
Related compounds
|
Titanium(III) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Titanium tetraiodide is an inorganic compound with the formula TiI4. It is a black volatile solid, first reported by Rudolph Weber in 1863.[2] It is an intermediate in the van Arkel–de Boer process for the purification of titanium.
- ^ "Titanium tetraiodide". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- ^ Weber, R. (1863). "Ueber die isomeren Modificationen der Titansäure und über einige Titanverbindungen". Annalen der Physik. 120 (10): 287–294. Bibcode:1863AnP...196..287W. doi:10.1002/andp.18631961003.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search