
Back حمض الترانيكساميك Arabic ترانکزامیک اسید AZB Asid tranecsamig Welsh Tranexamsäure German Τρανεξαμικό οξύ Greek Ácido tranexámico Spanish ترانکزامیک اسید Persian Traneksaamihappo Finnish Acide tranexamique French חומצה טראנקסאמית HE
 | |
| Clinical data | |
|---|---|
| Pronunciation | \ˌtran-eks-ˌam-ik-\ |
| Trade names | Cyklokapron, others |
| Other names | TXA |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612021 |
| License data | |
| Routes of administration | By mouth, intravenous, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 34% |
| Elimination half-life | 3.1 h |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.013.471 |
| Chemical and physical data | |
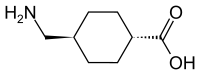
| Formula | C8H15NO2 |
| Molar mass | 157.213 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Tranexamic acid is a medication used to treat or prevent excessive blood loss from major trauma, postpartum bleeding, surgery, tooth removal, nosebleeds, and heavy menstruation.[6][7] It is also used for hereditary angioedema.[6][2] It is taken either by mouth, injection into a vein.[6], or by intramuscular injection.
Tranexamic acid is a synthetic analog of the amino acid lysine. It serves as an antifibrinolytic by reversibly binding four to five lysine receptor sites on plasminogen. This decreases the conversion of plasminogen to plasmin, preventing fibrin degradation and preserving the framework of fibrin's matrix structure.[4] Tranexamic acid has roughly eight times the antifibrinolytic activity of an older analogue, ε-aminocaproic acid.[citation needed] Tranexamic acid also directly inhibits the activity of plasmin with weak potency (IC50 = 87 mM),[8] and it can block the active-site of urokinase plasminogen activator (uPA) with high specificity (Ki = 2 mM), one of the highest among all the serine proteases.[9]
Side effects are rare.[2] Some include changes in color vision, seizures, blood clots, and allergic reactions.[2] Tranexamic acid appears to be safe for use during pregnancy and breastfeeding.[2][10] Tranexamic acid is an antifibrinolytic medication.[11]
Tranexamic acid was first made in 1962 by Japanese researchers Shosuke and Utako Okamoto.[12] It is on the World Health Organization's List of Essential Medicines.[13] Tranexamic acid is available as a generic drug.[14]
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Archived from the original on 6 July 2023. Retrieved 30 March 2024.
- ^ a b c d e "Cyklokapron Tablets - Summary of Product Characteristics (SPC) - (eMC)". emc. September 2016. Archived from the original on 20 December 2016. Retrieved 14 December 2016.
- ^ "Evana Heavy Period Relief Summary of Product Characteristics (SmPC)". (emc). 24 April 2024. Retrieved 14 May 2024.
- ^ a b Cite error: The named reference
Lysteda FDA labelwas invoked but never defined (see the help page). - ^ 会議事録 [Minutes of the meeting]. 薬事・食品衛生審議会一般用医薬品部会 (in Japanese). 22 March 2007. Archived from the original on 7 September 2022. Retrieved 7 September 2022.
- ^ a b c British national formulary: BNF 69 (69 ed.). British Medical Association. 2015. p. 170. ISBN 978-0-85711-156-2.
- ^ Shakur H, Roberts I, Fawole B, Chaudhri R, El-Sheikh M, Akintan A, et al. (WOMAN Trial Collaborators) (2017). "Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial". Lancet. 389 (10084): 2105–2116. doi:10.1016/S0140-6736(17)30638-4. PMC 5446563. PMID 28456509.
- ^ Law RH, Wu G, Leung EW, Hidaka K, Quek AJ, Caradoc-Davies TT, et al. (2017). "X-ray crystal structure of plasmin with tranexamic acid-derived active site inhibitors". Blood Advances. 1 (12): 766–771. doi:10.1182/bloodadvances.2016004150. PMC 5728053. PMID 29296720.
- ^ Wu G, Mazzitelli BA, Quek AJ, Veldman MJ, Conroy PJ, Caradoc-Davies TT, et al. (2019). "Tranexamic acid is an active site inhibitor of urokinase plasminogen activator". Blood Advances. 3 (5): 729–733. doi:10.1182/bloodadvances.2018025429. PMC 6418500. PMID 30814058.
- ^ "Tranexamic acid Use During Pregnancy". Drugs.com. Archived from the original on 21 December 2016. Retrieved 14 December 2016.
- ^ "Tranexamic Acid Injection - FDA prescribing information, side effects and uses". Drugs.com. Archived from the original on 21 December 2016. Retrieved 14 December 2016.
- ^ Watts G (2016). "Utako Okamoto". Lancet. 387 (10035): 2286. doi:10.1016/S0140-6736(16)30697-3. PMID 27308678.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 415. ISBN 978-1-284-05756-0.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search