
Back ثلاثي ميثيل أمين N-أكسيد Arabic تریومتیلآمین ان-اوکسید AZB Триметиламин N-оксид Bulgarian Trimethylaminoxid Czech Trimethylaminoxid German تریمتیلآمین ان-اکسید Persian Trimetyyliamiini-N-oksidi Finnish Oxyde de triméthylamine French Óxido de trimetilamina Galician Trimetilamina N-oksida ID
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Dimethylmethanamine N-oxide | |
| Other names
Trimethylamine oxide, TMAO, TMANO
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.341 |
| KEGG | |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
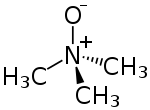
| C3H9NO | |
| Molar mass | 75.11 |
| Appearance | colorless solid |
| Melting point | 220 to 222 °C (428 to 432 °F; 493 to 495 K) (dihydrate: 96 °C) |
| good | |
| 5.4 D | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trimethylamine N-oxide (TMAO) is an organic compound with the formula (CH3)3NO. It is in the class of amine oxides. Although the anhydrous compound is known, trimethylamine N-oxide is usually encountered as the dihydrate. Both the anhydrous and hydrated materials are white, water-soluble solids.
TMAO is found in the tissues of marine crustaceans and marine fish, where it prevents water pressure from distorting proteins and thus killing the animal. The concentration of TMAO increases with the depth at which the animal lives; TMAO is found in high concentrations in the deepest-living described fish species, Pseudoliparis swirei, which was found in the Mariana Trench, at a recorded depth of 8,076 m (26,496 ft).[1][2]
In animals, TMAO is a product of the oxidation of trimethylamine, a common metabolite of trimethyl quaternary ammonium compounds, like choline, trimethylglycine, and L-carnitine.[3] High TMAO concentrations are associated with an increased risk of all-cause mortality and cardiovascular disease.[4][5][6]
- ^ Linley, T.D., M.E. Gerringer, P.H. Yancey, J.C. Drazen, C.L. Weinstock, A.J. Jamieson (2016). "Fishes of the hadal zone including new species, in situ observations and depth records of Liparidae". Deep Sea Research Part I: Oceanographic Research Papers. 114: 99–110. Bibcode:2016DSRI..114...99L. doi:10.1016/j.dsr.2016.05.003.
- ^ Gerringer, M.E., T.D. Linley, P.H. Yancey, A.J. Jamieson, E. Goetze, J.C. Drazen (2016). "Pseudoliparis swirei sp. nov.: A newly-discovered hadal snailfish (Scorpaeniformes: Liparidae) from the Mariana Trench". Zootaxa. 4358 (1): 161–177. doi:10.11646/zootaxa.4358.1.7. PMID 29245485.
- ^ Baker, J.R., Chaykin, S. (1 April 1962). "The biosynthesis of trimethylamine-N-oxide". J. Biol. Chem. 237 (4): 1309–13. doi:10.1016/S0021-9258(18)60325-4. PMID 13864146.
- ^ Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. (2017). "Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis". European Heart Journal. 38 (39): 2948–2956. doi:10.1093/eurheartj/ehx342. PMID 29020409.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Li D, Lu Y, Yuan S, Cai X, He Y, Chen J, Wu Q, He D, Fang A, Bo Y, Song P, Bogaert D, Tsilidis K, Larsson SC, Yu H, Zhu H, Theodoratou E, Zhu Y, Li X. (2022). "Gut microbiota-derived metabolite trimethylamine-N-oxide and multiple health outcomes: an umbrella review and updated meta-analysis". Am J Clin Nutr. 116 (1): 230–243. doi:10.1093/ajcn/nqac074. PMC 9257469. PMID 35348578.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dean YE, Rouzan SS, Loayza Pintado JJ, Talat NE, Mohamed ARH, Verma S, Anwar Kamdi Z, Gir D, Helmy A, Helmy Z, Afzal A, Mady T, Hazimeh Y, Aiash H. (2023). "Serum trimethylamine N-oxide levels among coronary artery disease and acute coronary syndrome patients: a systematic review and meta-analysis". Annals of Medicine and Surgery. 85 (12): 6123–6133. doi:10.1097/MS9.0000000000001426. PMC 10718322. PMID 38098555.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search