
Back Acetylacetonát kobaltitý Czech Tris-acétylacétonate de cobalt(III) French 乙酰丙酮钴(III) WUU 乙酰丙酮钴(III) Chinese
| |
| Names | |
|---|---|
| Other names
Cobalt(III) acetylacetonate, tris(acac) cobalt
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.040.464 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H21CoO6 | |
| Molar mass | 356.260 g·mol−1 |
| Appearance | green solid |
| Density | 1.41 g/cm3 |
| Melting point | 213 °C (415 °F; 486 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H317, H334 | |
| P261, P264, P270, P272, P280, P285, P301+P312, P302+P352, P304+P341, P321, P330, P333+P313, P342+P311, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
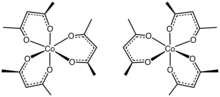
Tris(acetylacetonato)cobalt(III) is the coordination complex with the formula Co(C5H7O2)3. Often abbreviated Co(acac)3, it is a green, diamagnetic solid that is soluble in organic solvents, but not in water. Owing to its solubility in organic solvents, tris(acetylacetonato)cobalt(III) is used to produce homogeneous catalysts by reduction.[1]
- ^ Mayo, Peter D.; Tam, William (2002). "Tris(acetoacetonyl)cobalt". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00084. ISBN 0-471-93623-5.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search