
Back فوسفات ثلاثي الصوديوم Arabic تریسودیوم فوسفات AZB Fosfat de sodi Catalan Fosforečnan sodný Czech Trinatriumfosfat Danish Natriumphosphat German Natria fosfato Esperanto Fosfato trisódico Spanish تریسدیم فسفات Persian Natriumfosfaatti Finnish
| |
 Sodium, Na Phosphorus, P Oxygen, O | |
 Trisodium phosphate hydrate
| |
| Names | |
|---|---|
| IUPAC name
Trisodium phosphate
| |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.645 |
| EC Number |
|
| E number | E339(iii) (antioxidants, ...) |
| KEGG | |
PubChem CID
|
|
| RTECS number | |
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
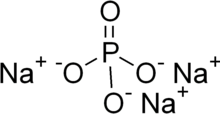
| Na3PO4 | |
| Molar mass | 163.939 g·mol−1 |
| Appearance | White, granular or crystalline solid |
| Density | 2.536 g/cm3 (17.5 °C, anhydrous) 1.62 g/cm3 (20 °C, dodecahydrate)[2][3][4] |
| Melting point | 1,583 °C (2,881 °F; 1,856 K) (anhydrous)[3] 73.4 °C (164.1 °F; 346.5 K) (dodecahydrate)[4] |
| Boiling point | 100 °C (212 °F; 373 K) (dodecahydrate) decomposes[4] |
| Solubility | Insoluble in ethanol, carbon disulfide[4] |
| Basicity (pKb) | 2.23 |
| Structure | |
| Trigonal | |
| Thermochemistry | |
Heat capacity (C)
|
665 J/(mol·K) (dodecahydrate)[4] |
Std molar
entropy (S⦵298) |
224.7 J/(mol·K) (anhydrous)[3] 660 J/(mol·K) (dodecahydrate)[4] |
Std enthalpy of
formation (ΔfH⦵298) |
−1935.5 kJ/mol (anhydrous)[3] −5480 kJ/mol (dodecahydrate)[4] |
Gibbs free energy (ΔfG⦵)
|
−1819 kJ/mol (anhydrous)[3] |
| Pharmacology | |
| A06AD17 (WHO) A06AG01 (WHO) B05XA09 (WHO) | |
| Hazards[6] | |
| GHS labelling: | |
 
| |
| Danger | |
| H315, H318, H335 | |
| P261, P280, P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | ICSC 1178 |
| Related compounds | |
Other cations
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trisodium phosphate (TSP) is an inorganic compound with the chemical formula Na3PO4. It is a white, granular or crystalline solid, highly soluble in water, producing an alkaline solution. TSP is used as a cleaning agent, builder, lubricant, food additive, stain remover, and degreaser.[7]
As an item of commerce TSP is often partially hydrated and may range from anhydrous Na3PO4 to the dodecahydrate Na3PO4·12H2O. Most often it is found in white powder form. It can also be called trisodium orthophosphate or simply sodium phosphate.
- ^ Merck Index, 12th Edition, 8808.
- ^ Eagleson, Mary, ed. (1994). Concise Encyclopedia Chemistry. Walter de Gruyter. p. 1000. ISBN 978-3-11-011451-5. Retrieved 25 May 2014.
- ^ a b c d e f "Sodium phosphate".
- ^ a b c d e f g h "Sodium phosphate dodecahydrate".
- ^ "Archived copy" (PDF). Archived from the original (PDF) on 14 March 2016. Retrieved 25 May 2014.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Sigma-Aldrich Co., Sodium phosphate. Retrieved on 2014-05-25.
- ^ Cite error: The named reference
Ullmannwas invoked but never defined (see the help page).
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
