Back Valien Afrikaans فالين Arabic والین AZB Валін Byelorussian Валін BE-X-OLD Валин Bulgarian ভ্যালিন Bengali/Bangla Valina Catalan Valin Czech Valin Danish
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Valine
| |||
| Other names
2-Amino-3-methylbutanoic acid
2-Aminoisovaleric acid Valic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider | |||
| DrugBank |
| ||
| ECHA InfoCard | 100.000.703 | ||
| EC Number |
| ||
| |||
| KEGG |
| ||
PubChem CID
|
|||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties[3] | |||
| C5H11NO2 | |||
| Molar mass | 117.148 g·mol−1 | ||
| Density | 1.316 g/cm3 | ||
| Melting point | 298 °C (568 °F; 571 K) (decomposition) | ||
| soluble, 85 g/L [1] | |||
| Acidity (pKa) | 2.32 (carboxyl), 9.62 (amino)[2] | ||
| -74.3·10−6 cm3/mol | |||
| Supplementary data page | |||
| Valine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
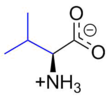
Valine (symbol Val or V)[4] is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isopropyl group, making it a non-polar aliphatic amino acid. Valine is essential in humans, meaning the body cannot synthesize it; it must be obtained from dietary sources which are foods that contain proteins, such as meats, dairy products, soy products, beans and legumes. It is encoded by all codons starting with GU (GUU, GUC, GUA, and GUG).
- ^ "Physicochemical Information". emdmillipore. 2022. Retrieved 17 November 2022.
- ^ Dawson RM, Elliott DC, Elliott WH, Jones KM, eds. (1959). Data for Biochemical Research. Oxford: Clarendon Press. ASIN B000S6TFHA. OCLC 859821178.
- ^ Weast RC, ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-569. ISBN 0-8493-0462-8.
- ^ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search