
Back Neuraminidase (Influenzavirus A) German Neuraminidase virale French Virusna neuraminidaza Croatian ウイルス・ノイラミニダーゼ Japanese
| Neuraminidase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
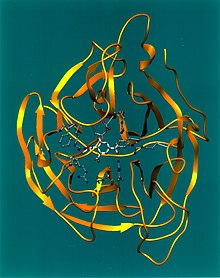 Crystallographic structure of influenza neuraminidase in complex with the inhibitor 4-acetamido-3-hydroxy-5-nitro-benzoic acid.[1] | |||||||||||
| Identifiers | |||||||||||
| Symbol | Neur | ||||||||||
| Pfam | PF00064 | ||||||||||
| Pfam clan | CL0434 | ||||||||||
| InterPro | IPR001860 | ||||||||||
| SCOP2 | 2bat / SCOPe / SUPFAM | ||||||||||
| CAZy | GH34 | ||||||||||
| CDD | cd00260 | ||||||||||
| |||||||||||


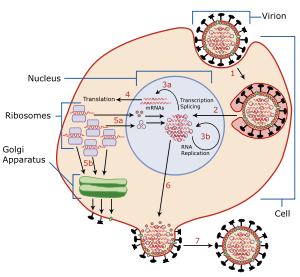
Viral neuraminidase is a type of neuraminidase found on the surface of influenza viruses that enables the virus to be released from the host cell. Neuraminidases are enzymes that cleave sialic acid (also called neuraminic acid) groups from glycoproteins. Viral neuraminidase was discovered by Alfred Gottschalk at the Walter and Eliza Hall Institute in 1957.[3] Neuraminidase inhibitors are antiviral agents that inhibit influenza viral neuraminidase activity and are of major importance in the control of influenza.[4]
Viral neuraminidases are the members of the glycoside hydrolase family 34 CAZY GH_34 which comprises enzymes with only one known activity; sialidase or neuraminidase EC 3.2.1.18. Neuraminidases cleave the terminal sialic acid residues from carbohydrate chains in glycoproteins. Sialic acid is a negatively charged sugar associated with the protein and lipid portions of lipoproteins.[citation needed]
To infect a host cell, the influenza virus attaches to the exterior cell surface using hemagglutinin, a molecule found on the surface of the virus that binds to sialic acid groups. Sialic acids are found on various glycoproteins at the host cell surface. The virus then moves from sialic acid group to sialic acid group until it finds the proper cell surface receptor (whose identity remains unknown).[5] Neuraminadase enables this movement by cleaving sialic acid groups that hemagglutinin was attached to. After the virus has entered the cell and has replicated, new viral particles bud from the host cell membrane. The hemagglutinin on new viral particles remains attached to sialic acid groups of glycoproteins on the external cell surface and the surface of other viral particles; neuraminadase cleaves these groups and thereby allows the release of viral particles[6] and prevents self-aggregation.[5] Neuraminadase also facilitates the movement of virus particles in the presence of mucus rich in silicic acid.[5]
A single hemagglutinin-neuraminidase protein can combine neuraminidase and hemagglutinin functions, such as in mumps virus and human parainfluenza virus.[citation needed]
- ^ Jedrzejas, MJ; Singh, S; Brouillette, WJ; Laver, WG; Air, GM; Luo, M (14 March 1995). "Structures of aromatic inhibitors of influenza virus neuraminidase". Biochemistry. 34 (10): 3144–51. doi:10.1021/bi00010a003. PMID 7880809.
- ^ Varghese, J. N.; McKimm-Breschkin, J. L.; Caldwell, J. B.; Kortt, A. A.; Colman, P. M. (1992). "The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor". Proteins: Structure, Function, and Genetics. 14 (3): 327–32. doi:10.1002/prot.340140302. PMID 1438172. S2CID 41743465.
- ^ "WEHI History: 1957 Discovery of Neuraminidase - Key Flu Molecule". WEHI. Retrieved 2023-11-08.
- ^ Couch RB (1999). "Measures for control of influenza". PharmacoEconomics. 16 (Suppl 1): 41–5. doi:10.2165/00019053-199916001-00006. PMID 10623375. S2CID 41844816.
- ^ a b c Dou, Dan; Revol, Rebecca; Östbye, Henrik; Wang, Hao; Daniels, Robert (2018-07-20). "Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement". Frontiers in Immunology. 9: 1581. doi:10.3389/fimmu.2018.01581. ISSN 1664-3224. PMC 6062596. PMID 30079062.
- ^ Huang IC, Li W, Sui J, Marasco W, Choe H, Farzan M (May 2008). "Influenza A virus neuraminidase limits viral superinfection". J. Virol. 82 (10): 4834–43. doi:10.1128/JVI.00079-08. PMC 2346733. PMID 18321971.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search