
Back Sinknitried Afrikaans نتريد الزنك Arabic نیترید چینکو AZB Zinknitrid German Nitruro de zinc Spanish نیترید روی Persian Nitruro di zinco Italian 窒化亜鉛 Japanese സിങ്ക് നൈട്രൈഡ് Malayalam Нитрид цинка Russian
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.013.826 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Zn3N2 | |
| Molar mass | 224.154 g/mol |
| Appearance | blue-gray cubic crystals[1] |
| Density | 6.22 g/cm3, solid[1] |
| Melting point | decomposes 700°C[1] |
| insoluble, reacts | |
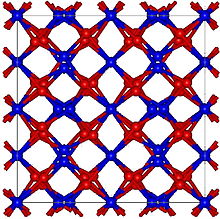
| Structure | |
| Cubic, cI80 | |
| Ia-3, No. 206[2] | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319 | |
| P264, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Zinc nitride (Zn3N2) is an inorganic compound of zinc and nitrogen, usually obtained as (blue)grey crystals. It is a semiconductor. In pure form, it has the anti-bixbyite structure.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
