
Back زولبيديم Arabic زولپیدم AZB Zolpidem Czech Solpidem Welsh Zolpidem German Ζολπιδέμη Greek Zolpidem Spanish زولپیدم Persian Tsolpideemi Finnish Zolpidem French
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ambien and Ambien CR, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693025 |
| License data | |
| Pregnancy category |
|
| Dependence liability | Physical: High Psychological: Moderate[3] |
| Addiction liability | High[4] |
| Routes of administration | By mouth, sublingual, oromucosal (spray), rectal |
| Drug class | Nonbenzodiazepine, sedative-hypnotic |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 70% (by mouth) |
| Protein binding | 92%[9] |
| Metabolism | Liver through CYP3A4 (~60%), CYP2C9 (~20%), and CYP1A2 (~14%)[11] |
| Metabolites | (ZCA) zolpidem 6-carboxylic acid; (ZPCA) zolpidem phenyl-4-carboxylic acid |
| Onset of action | ≤ 30 Minutes |
| Elimination half-life | 2.0 - 3 hours[10][9] |
| Duration of action | 3 hours |
| Excretion | Kidney (56%) fecal (34%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.115.604 |
| Chemical and physical data | |
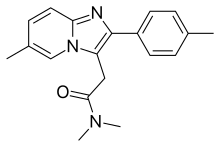
| Formula | C19H21N3O |
| Molar mass | 307.397 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 193–197 °C (379–387 °F) [9] |
| |
| |
| | |
Zolpidem, sold under the brand name Ambien among others, is a medication primarily used for the short-term treatment of sleeping problems.[10][12] Guidelines recommend that it be used only after cognitive behavioral therapy for insomnia and after behavioral changes, such as sleep hygiene, have been tried.[13][14][15] It decreases the time to sleep onset by about fifteen minutes and at larger doses helps people stay asleep longer.[7] It is taken by mouth and is available in conventional tablets, sublingual tablets, or oral spray.[10]
Common side effects include daytime sleepiness, headache, nausea, and diarrhea.[10] More severe side effects include memory problems and hallucinations.[7] While flumazenil, a GABAA–receptor antagonist, can reverse zolpidem's effects, usually supportive care is all that is recommended in overdose.[16]
Zolpidem is a nonbenzodiazepine or Z-drug which acts as a sedative and hypnotic.[10][16] Zolpidem is a GABAA receptor agonist of the imidazopyridine class.[10] It works by increasing GABA effects in the central nervous system by binding to GABAA receptors at the same location as benzodiazepines.[10] It generally has a half-life of two to three hours.[10] This, however, is increased in those with liver problems.[10]
Zolpidem was approved for medical use in the United States in 1992.[10][17] It became available as a generic medication in 2007.[18] Zolpidem is a Schedule IV controlled substance under the Controlled Substances Act of 1970 (CSA).[7][8] More than ten million prescriptions are filled each year in the United States, making it one of the most commonly used treatments for sleeping problems.[19][20] In 2021, it was the 63rd most commonly prescribed medication in the United States, with more than 10 million prescriptions.[21][22]
- ^ Cite error: The named reference
genericnameswas invoked but never defined (see the help page). - ^ "Zolpidem Use During Pregnancy". Drugs.com. 30 June 2020. Retrieved 14 August 2020.
- ^ Ries RK (2009). Principles of addiction medicine (4 ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. p. 106. ISBN 978-0-7817-7477-2. Archived from the original on 8 September 2017.
- ^ Victorri-Vigneau C, Gérardin M, Rousselet M, Guerlais M, Grall-Bronnec M, Jolliet P (January 2014). "An update on zolpidem abuse and dependence". Journal of Addictive Diseases. 33 (1): 15–23. doi:10.1080/10550887.2014.882725. ISSN 1055-0887. PMID 24467433.
- ^ "Scheduling of zolpidem (Stilnox)". Therapeutic Goods Administration (TGA). 21 February 2008. Retrieved 15 August 2020.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 3 August 2023.
- ^ a b c d Matheson E, Hainer BL (July 2017). "Insomnia: Pharmacologic Therapy". American Family Physician. 96 (1): 29–35. PMID 28671376.
- ^ a b "Ambien- zolpidem tartrate tablet, film coated". DailyMed. 29 August 2019. Retrieved 15 August 2020.
- ^ a b c Salvà P, Costa J (September 1995). "Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications". Clinical Pharmacokinetics. 29 (3): 142–153. doi:10.2165/00003088-199529030-00002. PMID 8521677. S2CID 23391285.
- ^ a b c d e f g h i j "Zolpidem (Monograph)". The American Society of Health-System Pharmacists. 27 April 2023. Retrieved 10 March 2024.
- ^ Von Moltke LL, Greenblatt DJ, Granda BW, Duan SX, Grassi JM, Venkatakrishnan K, et al. (July 1999). "Zolpidem metabolism in vitro: responsible cytochromes, chemical inhibitors, and in vivo correlations". British Journal of Clinical Pharmacology. 48 (1): 89–97. doi:10.1046/j.1365-2125.1999.00953.x. PMC 2014868. PMID 10383565.
- ^ Cite error: The named reference
UKlabelwas invoked but never defined (see the help page). - ^ Cite error: The named reference
NICE2014was invoked but never defined (see the help page). - ^ Cite error: The named reference
EUsleep2017was invoked but never defined (see the help page). - ^ Cite error: The named reference
ACP2016was invoked but never defined (see the help page). - ^ a b Gunja N (June 2013). "The clinical and forensic toxicology of Z-drugs". Journal of Medical Toxicology. 9 (2): 155–62. doi:10.1007/s13181-013-0292-0. PMC 3657020. PMID 23404347.
- ^ "Drug Approval Package: Ambien (Zolpidem Tartrate) NDA 19908". U.S. Food and Drug Administration (FDA). 24 December 1999. Retrieved 15 August 2020.
- ^ "FDA Approves First Generic Versions of Ambien (Zolpidem Tartrate) for the Treatment of Insomnia". U.S. Food and Drug Administration (FDA) (Press release). Archived from the original on 6 March 2010. Retrieved 24 January 2010.
- ^ "Zolpidem". LiverTox. Archived from the original on 16 March 2018. Retrieved 15 March 2018.
- ^ "Some Sleep Drugs Can Impair Driving". U.S. Food and Drug Administration (FDA). 13 June 2013. Retrieved 15 March 2018.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Zolpidem - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search