
Back Aktiveringsenergie Afrikaans طاقة تنشيط Arabic Aktivləşmə enerjisi Azerbaijani Енергия на активация Bulgarian সক্রিয়ন শক্তি Bengali/Bangla སད་སློང་ནུས་པ། Tibetan Energija aktivacije BS Energia d'activació Catalan وزەی چالاککردن CKB Aktivační energie Czech
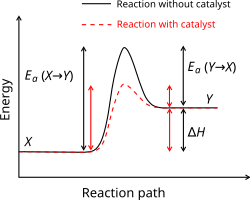
The activation energy of a chemical reaction is the minimum energy that is needed to make the reaction happen. It usually has the symbol Ea and it is measured in kilojoule per mole. It can be thought of as a barrier between the reagents and the products of a reaction. The activation energy is the difference in energy between the transition state and the starting reagents.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search