
Back Kafeïen Afrikaans كافيين Arabic كافيين ARZ Cafeína AST Kofein Azerbaijani Кафеін Byelorussian Кафэін BE-X-OLD Кофеин Bulgarian ক্যাফেইন Bengali/Bangla Kofein BS
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /kæˈfiːn, ˈkæfiːn/ |
| Synonyms | Guaranine Methyltheobromine 1,3,7-Trimethylxanthine 7-methyltheophylline[6] Theine |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Dependence liability | Physical: low–moderate[1][2][3][4] Psychological: low[5] |
| Addiction liability | Low[4] / none[1][2][3] |
| Routes of administration | By mouth, insufflation, enema, rectal, intravenous |
| Drug class | Stimulant |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 99%[8] |
| Protein binding | 25–36%[7] |
| Metabolism | Primary: CYP1A2[7] Minor: CYP2E1,[7] CYP3A4,[7] CYP2C8,[7] CYP2C9[7] |
| Metabolites | Paraxanthine (84%) Theobromine (12%) Theophylline (4%) |
| Onset of action | ~1 hour[8] |
| Elimination half-life | Adults: 3–7 hours[7] Infants (full term): 8 hours[7] Infants (premature): 100 hours[7] |
| Duration of action | 3–4 hours[8] |
| Excretion | Urine (100%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.000.329 |
| Chemical and physical data | |
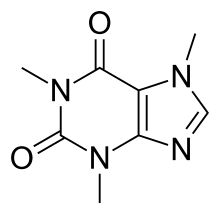
| Formula | C8H10N4O2 |
| Molar mass | 194.19 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.23 g/cm3 |
| Melting point | 235 to 238 °C (455 to 460 °F) (anhydrous)[9][10] |
| |
| |

Caffeine is a central nervous system (CNS) stimulant. It is in the methylxanthine class. It is found in many plants, and is used in drinks like coffee, some teas, some soft drinks, and energy drinks. It can be harmful for both humans and animals if a large amount is consumed. If a person ate 10-13 grams of caffeine quickly, between 80 and 100 cups of coffee, they would overdose and may die.[11] It is the world's most popular psychoactive drug, and is legal in nearly all of the world.[12]
- ↑ 1.0 1.1 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 375. ISBN 978-0-07-148127-4.
Long-term caffeine use can lead to mild physical dependence. A withdrawal syndrome characterized by drowsiness, irritability, and headache typically lasts no longer than a day. True compulsive use of caffeine has not been documented.
- ↑ 2.0 2.1 Karch SB (2009). Karch's pathology of drug abuse (4th ed.). Boca Raton: CRC Press. pp. 229–230. ISBN 978-0-8493-7881-2.
- ↑ 3.0 3.1 American Psychiatric Association (2013). "Substance-Related and Addictive Disorders" (PDF). American Psychiatric Publishing. pp. 1–2. Archived from the original (PDF) on 15 August 2015. Retrieved 10 July 2015.
Substance use disorder in DSM-5 combines the DSM-IV categories of substance abuse and substance dependence into a single disorder measured on a continuum from mild to severe. ... Additionally, the diagnosis of dependence caused much confusion. Most people link dependence with "addiction" when in fact dependence can be a normal body response to a substance. ... DSM-5 will not include caffeine use disorder, although research shows that as little as two to three cups of coffee can trigger a withdrawal effect marked by tiredness or sleepiness. There is sufficient evidence to support this as a condition, however it is not yet clear to what extent it is a clinically significant disorder.
- ↑ 4.0 4.1 Introduction to Pharmacology (third ed.). Abingdon: CRC Press. 2007. pp. 222–223. ISBN 978-1-4200-4742-4.
- ↑ Juliano LM, Griffiths RR (October 2004). "A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features". Psychopharmacology. 176 (1): 1–29. doi:10.1007/s00213-004-2000-x. PMID 15448977. S2CID 5572188.
- ↑ "Caffeine". ChemSpider. Retrieved 16 November 2021.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 "Caffeine". DrugBank. University of Alberta. 16 September 2013. Retrieved 8 August 2014.
- ↑ 8.0 8.1 8.2 Poleszak E, Szopa A, Wyska E, Kukuła-Koch W, Serefko A, Wośko S, Bogatko K, Wróbel A, Wlaź P (February 2016). "Caffeine augments the antidepressant-like activity of mianserin and agomelatine in forced swim and tail suspension tests in mice". Pharmacological Reports. 68 (1): 56–61. doi:10.1016/j.pharep.2015.06.138. PMID 26721352. S2CID 19471083.
- ↑ "Caffeine". Pubchem Compound. NCBI. Retrieved 16 October 2014.
Boiling Point
178 °C (sublimes)
Melting Point
238 DEG C (ANHYD) - ↑ "Caffeine". ChemSpider. Royal Society of Chemistry. Retrieved 16 October 2014.
Experimental Melting Point:
234–236 °C Alfa Aesar
237 °C Oxford University Chemical Safety Data
238 °C LKT Labs [C0221]
237 °C Jean-Claude Bradley Open Melting Point Dataset 14937
238 °C Jean-Claude Bradley Open Melting Point Dataset 17008, 17229, 22105, 27892, 27893, 27894, 27895
235.25 °C Jean-Claude Bradley Open Melting Point Dataset 27892, 27893, 27894, 27895
236 °C Jean-Claude Bradley Open Melting Point Dataset 27892, 27893, 27894, 27895
235 °C Jean-Claude Bradley Open Melting Point Dataset 6603
234–236 °C Alfa Aesar A10431, 39214
Experimental Boiling Point:
178 °C (Sublimes) Alfa Aesar
178 °C (Sublimes) Alfa Aesar 39214 - ↑ "Can caffeine kill you?". Livescience.com. 25 September 2012. Retrieved 19 February 2016.
- ↑ Burchfield G (1997). Meredith H (ed.). "What's your poison: caffeine". Australian Broadcasting Corporation. Archived from the original on 26 July 2009. Retrieved 15 January 2014.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search