Back Etanol Afrikaans Ethanol ALS Etanol AN إيثانول Arabic ইথান'ল Assamese Etanol AST दारु AWA Etanol Azerbaijani اتانول AZB Этанол Bashkir
| |||
| |||
| Names | |||
|---|---|---|---|
| Pronunciation | /ˈɛθənɒl/ | ||
| Systematic IUPAC name
ethanol[1] | |||
| Other names
Absolute alcohol
alcohol cologne spirit drinking alcohol ethylic alcohol EtOH ethyl alcohol ethyl hydrate ethyl hydroxide ethylol grain alcohol hydroxyethane methylcarbinol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| Beilstein Reference | 1718733 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.526 | ||
| Gmelin Reference | 787 | ||
PubChem CID
|
|||
| UNII | |||
| UN number | UN 1170 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
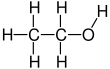
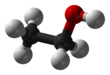
| C2H6O | |||
| Molar mass | 46.07 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.7893 g/cm3 (at 20 °C)[2] | ||
| Melting point | −114.14 ± 0.03[2] °C (−173.45 ± 0.05 °F; 159.01 ± 0.03 K) | ||
| Boiling point | 78.24 ± 0.09[2] °C (172.83 ± 0.16 °F; 351.39 ± 0.09 K) | ||
| miscible | |||
| log P | −0.18 | ||
| Vapor pressure | 5.95 kPa (at 20 °C) | ||
| Acidity (pKa) | 15.9 (H2O), 29.8 (DMSO)[3][4] | ||
| −33.60·10−6 cm3/mol | |||
Refractive index (nD)
|
1.3611[2] | ||
| Viscosity | 1.2 mPa·s (at 20 °C), 1.074 mPa·s (at 25 °C)[5] | ||
| 1.69 D[6] | |||
| Hazards | |||
| NFPA 704 |
| ||
| Flash point | 14 °C (Absolute) | ||
| U.S. Permissible exposure limit (PEL) |
TWA 1000 ppm (1900 mg/m3) [7] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||

Ethanol, also known as ethyl alcohol, grain alcohol or just alcohol, is a flammable, colorless chemical compound. Its chemical formula is C2H5OH, also written as C2H6O. It is the active part of alcoholic drinks, which are drunk in most cultures worldwide. It is also used as a solvent because it can dissolve many other chemicals and is not very toxic. Yeast makes most of the ethanol that people use.
- ↑ "Ethanol – Compound Summary". The PubChem Project. USA: National Center for Biotechnology Information.
- ↑ 2.0 2.1 2.2 2.3 Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 3.246. ISBN 1439855110.
- ↑ Ballinger P, Long FA (1960). "Acid Ionization Constants of Alcohols. II. Acidities of Some Substituted Methanols and Related Compounds1,2". Journal of the American Chemical Society. 82 (4): 795–798. doi:10.1021/ja01489a008.
- ↑ Arnett EM, Venkatasubramaniam KG (1983). "Thermochemical acidities in three superbase systems". J. Org. Chem. 48 (10): 1569–1578. doi:10.1021/jo00158a001.
- ↑ Lide DR, ed. (2012). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL.: CRC Press/Taylor and Francis. pp. 6–232. ISBN 9781439855126.
- ↑ Lide DR, ed. (2008). CRC Handbook of Chemistry and Physics (89th ed.). Boca Raton: CRC Press. pp. 9–55. ISBN 9781420066791.
- ↑ NIOSH Pocket Guide to Chemical Hazards. "#0262". National Institute for Occupational Safety and Health (NIOSH).
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search