
Back نظائر الهيدروجين Arabic Isótopos del hidróxenu AST হাইড্রোজেন আইসোটোপসমূহ Bengali/Bangla Isòtops de l'hidrogen Catalan Izotopy vodíku Czech Водород изотопĕсем CV Isotopes of hydrogen English Izotopoj de hidrogeno Esperanto Anexo:Isótopos de hidrógeno Spanish Vesiniku isotoobid Estonian
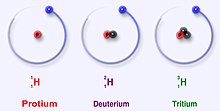
Hydrogen has three main isotopes; protium (1H), deuterium (2H) and tritium (3H). These isotopes form naturally in nature. Protium and deuterium are stable. Tritium is radioactive and has a half-life of about 12 years. Scientists have created four other hydrogen isotopes (4H to 7H), but these isotopes are very unstable and do not exist naturally.
The main isotopes of hydrogen are unique because they are the only isotopes that have a name. These names are still in use today. Deuterium and tritium sometimes get their own symbols, D and T. However, the International Union of Pure and Applied Chemistry does not like these names much, even though they are often used. There are other isotopes that had their own names when scientists studied radioactivity. But, their names are no longer used today.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search