Back Аӡы Abkhazian Water Afrikaans Wasser ALS ውሃ Amharic Augua AN Wæter ANG ماء Arabic ܡܝܐ ARC ما ARY ميه ARZ
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
water, oxidane
| |||
| Other names | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| Beilstein Reference | 3587155 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.902 | ||
| Gmelin Reference | 117 | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
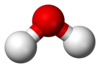
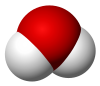
| H 2O | |||
| Molar mass | 18.01528(33) g/mol | ||
| Appearance | White crystal-like solid, almost colorless liquid with a hint of blue, colorless gas | ||
| Odor | None | ||
| Density | Liquid:[3] 0.9998396 g/mL at 0 °C 0.9970474 g/mL at 25 °C 0.961893 g/mL at 95 °C Solid:[4] 0.9167 g/ml at 0 °C | ||
| Melting point | 0.00 °C (32.00 °F; 273.15 K) [a] | ||
| Boiling point | 99.98 °C (211.96 °F; 373.13 K) [5][a] | ||
| N/A | |||
| Solubility | Poorly soluble in haloalkanes, aliphatic and aromatic hydrocarbons, ethers.[6] Improved solubility in carboxylates, alcohols, ketones, amines. Miscible with methanol, ethanol, propanol, isopropanol, acetone, glycerol, 1,4-dioxane, tetrahydrofuran, sulfolane, acetaldehyde, dimethylformamide, dimethoxyethane, dimethyl sulfoxide, acetonitrile. Partially miscible with Diethyl ether, Methyl Ethyl Ketone, Dichloromethane, Ethyl Acetate, Bromine. | ||
| Vapor pressure | 3.1690 kilopascals or 0.031276 atm[7] | ||
| Acidity (pKa) | 13.995[8][9][b] | ||
| Basicity (pKb) | 13.995 | ||
| Conjugate acid | Hydronium | ||
| Conjugate base | Hydroxide | ||
| Thermal conductivity | 0.6065 W/(m·K)[12] | ||
Refractive index (nD)
|
1.3330 (20 °C)[13] | ||
| Viscosity | 0.890 cP[14] | ||
| Structure | |||
| Hexagonal | |||
| C2v | |||
| Bent | |||
| 1.8546 D[15] | |||
| Thermochemistry | |||
| Std enthalpy of formation ΔfH |
−285.83 ± 0.04 kJ/mol[6][16] | ||
| Standard molar entropy S |
69.95 ± 0.03 J/(mol·K)[16] | ||
| Specific heat capacity, C | 75.385 ± 0.05 J/(mol·K)[16] | ||
| Hazards | |||
| Main hazards | Drowning Avalanche (as snow)
| ||
| NFPA 704 |
| ||
| Flash point | Non-flammable | ||
| Related compounds | |||
| Other cations | Hydrogen sulfide Hydrogen selenide Hydrogen telluride Hydrogen polonide Hydrogen peroxide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||

Water (H
2O) is a transparent, tasteless, odourless, and almost always colourless chemical substance and covers about 71% of Earth's surface.
No known life can live without it. Water is essential for life.[17] This has to be qualified a bit. There are some forms of life which can survive without it, but cannot reproduce without it. Since reproduction is a central part of life, it is clear that water is essential for an organism to survive and reproduce.
Lakes, oceans, seas, and rivers are made of water. Precipitation is water that falls from clouds in the sky. It may be rain if it is liquid, or it may be snow or ice frozen if it is cold. When water gets below 0 °C (32 °F), it freezes and becomes ice, the frozen kind of water. If water gets very hot (above 100 °C (212 °F), it boils and becomes steam or water vapor.
There is a water cycle.
- ↑ "naming molecular compounds". www.iun.edu. Archived from the original on 24 September 2018. Retrieved 1 October 2018.
Sometimes these compounds have generic or common names (e.g., H2O is "water") and they also have systematic names (e.g., H2O, dihydrogen monoxide).
- ↑ "Definition of Hydrol". Merriam-Webster.
- ↑ Riddick 1970, Table of Physical Properties, Water 0b. pg 67-8.
- ↑ Lide 2003, Properties of Ice and Supercooled Water in Section 6.
- ↑ Water in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2016-5-27)
- ↑ 6.0 6.1 Anatolievich, Kiper Ruslan. "Properties of substance: water". Archived from the original on 2014-06-02. Retrieved 2021-02-07.
- ↑ Lide 2003, Vapor Pressure of Water From 0 to 370° C in Sec. 6.
- ↑ Lide 2003, Chapter 8: Dissociation Constants of Inorganic Acids and Bases.
- ↑ Weingärtner et al. 2016, p. 13.
- ↑ "What is the pKa of Water". University of California, Davis. 2015-08-09.
- ↑ Silverstein, Todd P.; Heller, Stephen T. (17 April 2017). "pKa Values in the Undergraduate Curriculum: What Is the Real pKa of Water?". Journal of Chemical Education. 94 (6): 690–695. Bibcode:2017JChEd..94..690S. doi:10.1021/acs.jchemed.6b00623.
- ↑ Ramires, Maria L. V.; Castro, Carlos A. Nieto de; Nagasaka, Yuchi; Nagashima, Akira; Assael, Marc J.; Wakeham, William A. (1995-05-01). "Standard Reference Data for the Thermal Conductivity of Water". Journal of Physical and Chemical Reference Data. 24 (3): 1377–1381. Bibcode:1995JPCRD..24.1377R. doi:10.1063/1.555963. ISSN 0047-2689.
- ↑ Lide 2003, 8—Concentrative Properties of Aqueous Solutions: Density, Refractive Index, Freezing Point Depression, and Viscosity.
- ↑ Lide 2003, 6.186.
- ↑ Lide 2003, 9—Dipole Moments.
- ↑ 16.0 16.1 16.2 Water in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2014-06-01)
- ↑ "United Nations". Un.org. 2005-03-22. Retrieved 2010-07-25.
<ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search