
Back Selbstorganisierende Monoschicht German Αυτοσυναρμολογούμενες μονοστιβάδες Greek Monocapa autoensamblada Spanish تک لایههای خودآرا Persian Monostrato auto-assemblato Italian 自己組織化単分子膜 Japanese Самособирающиеся монослои Russian
| Part of a series of articles on |
| Molecular self-assembly |
|---|
 |
Self-assembled monolayers (SAM) of organic molecules are molecular assemblies formed spontaneously on surfaces by adsorption and are organized into more or less large ordered domains.[1][2] In some cases molecules that form the monolayer do not interact strongly with the substrate. This is the case for instance of the two-dimensional supramolecular networks[3] of e.g. perylenetetracarboxylic dianhydride (PTCDA) on gold[4] or of e.g. porphyrins on highly oriented pyrolitic graphite (HOPG).[5] In other cases the molecules possess a head group that has a strong affinity to the substrate and anchors the molecule to it.[6] Such a SAM consisting of a head group, tail and functional end group is depicted in Figure 1. Common head groups include thiols, silanes, phosphonates, etc.
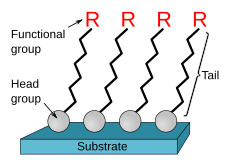
SAMs are created by the chemisorption of "head groups" onto a substrate from either the vapor or liquid phase[7][8] followed by a slow organization of "tail groups".[9] Initially, at small molecular density on the surface, adsorbate molecules form either a disordered mass of molecules or form an ordered two-dimensional "lying down phase",[7] and at higher molecular coverage, over a period of minutes to hours, begin to form three-dimensional crystalline or semicrystalline structures on the substrate surface.[10] The "head groups" assemble together on the substrate, while the tail groups assemble far from the substrate. Areas of close-packed molecules nucleate and grow until the surface of the substrate is covered in a single monolayer.
Adsorbate molecules adsorb readily because they lower the surface free-energy of the substrate[1] and are stable due to the strong chemisorption of the "head groups." These bonds create monolayers that are more stable than the physisorbed bonds of Langmuir–Blodgett films.[11][12] A trichlorosilane based "head group", for example in a FDTS molecule, reacts with a hydroxyl group on a substrate, and forms very stable, covalent bond [R-Si-O-substrate] with an energy of 452 kJ/mol. Thiol-metal bonds are on the order of 100 kJ/mol, making them fairly stable in a variety of temperatures, solvents, and potentials.[10] The monolayer packs tightly due to van der Waals interactions,[1][12] thereby reducing its own free energy.[1] The adsorption can be described by the Langmuir adsorption isotherm if lateral interactions are neglected. If they cannot be neglected, the adsorption is better described by the Frumkin isotherm.[10]
- ^ a b c d Love; et al. (2005). "Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology". Chem. Rev. 105 (4): 1103–1170. doi:10.1021/cr0300789. PMID 15826011.
- ^ Barlow, S.M.; Raval R.. (2003). "Complex organic molecules at metal surfaces: bonding, organisation and chirality". Surface Science Reports. 50 (6–8): 201–341. Bibcode:2003SurSR..50..201B. doi:10.1016/S0167-5729(03)00015-3.
- ^ Elemans, J.A.A.W.; Lei S., De Feyter S. (2009). "Molecular and Supramolecular Networks on Surfaces: From Two-Dimensional Crystal Engineering to Reactivity". Angew. Chem. Int. Ed. 48 (40): 7298–7332. doi:10.1002/anie.200806339. hdl:2066/75325. PMID 19746490.
- ^ Witte, G.; Wöll Ch. (2004). "Growth of aromatic molecules on solid substrates for applications in organic electronics". Journal of Materials Research. 19 (7): 1889–1916. Bibcode:2004JMatR..19.1889W. doi:10.1557/JMR.2004.0251.
- ^ De Feyter, S.; De Schreyer F.C. (2003). "Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy". Chemical Society Reviews. 32 (3): 139–150. CiteSeerX 10.1.1.467.5727. doi:10.1039/b206566p. PMID 12792937.
- ^ Carroll, Gregory T.; Pollard, Michael M.; van Delden, Richard A.; Feringa, Ben L. (2010). "Controlled rotary motion of light-driven molecular motors assembled on a gold film" (PDF). Chem. Sci. 1 (1): 97–101. doi:10.1039/C0SC00162G. S2CID 97346507.
- ^ a b Schwartz, D.K., Mechanisms and Kinetics of Self-Assembled Monolayer Formation (2001). "Mechanisms and kinetics of self-assembled monolayer formation". Annu. Rev. Phys. Chem. 52: 107–37. Bibcode:2001ARPC...52..107S. doi:10.1146/annurev.physchem.52.1.107. PMID 11326061.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schreiber, F (30 November 2000). "Structure and growth of self-assembling monolayers". Progress in Surface Science. 65 (5–8): 151–257. Bibcode:2000PrSS...65..151S. doi:10.1016/S0079-6816(00)00024-1.
- ^ Wnek, Gary, Gary L. Bowlin (2004). Encyclopedia of Biomaterials and Biomedical Engineering. Informa Healthcare. pp. 1331–1333.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c Vos, Johannes G., Robert J. Forster, Tia E. Keyes (2003). Interfacial Supramolecular Assemblies. Wiley. pp. 88–94.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Madou, Marc (2002). Fundamentals of Microfabrication: The Science of Miniaturization. CRC. pp. 62–63.
- ^ a b Kaifer, Angel (2001). Supramolecular Electrochemistry. Coral Gables. Wiley VCH. pp. 191–193.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search