
Back Seep Afrikaans ሳሙና Amharic Sabón AN صابون Arabic صابون ARZ চাবোন Assamese Xabón AST Jawuna Aymara Sabun Azerbaijani Һабын Bashkir
This article is missing information about What determines hardness of soap and solubility to water and oil, why dissolving speed varies. (April 2024) |

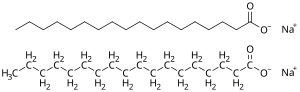

Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products.[1] In a domestic setting, soaps are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are used as thickeners, components of some lubricants, and precursors to catalysts.
When used for cleaning, soap solubilizes particles and grime, which can then be separated from the article being cleaned. In hand washing, as a surfactant, when lathered with a little water, soap kills microorganisms by disorganizing their membrane lipid bilayer and denaturing their proteins.[citation needed] It also emulsifies oils, enabling them to be carried away by running water.[2]
Soap is created by mixing fats and oils with a base.[3] Humans have used soap for millennia; evidence exists for the production of soap-like materials in ancient Babylon around 2800 BC.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Soap". doi:10.1351/goldbook.S05721
- ^ Tumosa, Charles S. (2001-09-01). "A Brief History of Aluminum Stearate as a Component of Paint". cool.conservation-us.org. Archived from the original on 2017-03-18. Retrieved 2017-04-05.
- ^ "What's The Difference Between Soap and Detergent". cleancult.com. Archived from the original on 2019-12-18. Retrieved 2019-12-18.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search