Back Metaantiol Afrikaans ميثانثيول Arabic متانتیول AZB Metanotiol Catalan Methylmerkaptan Czech Methanthiol German Μεθανοθειόλη Greek Metanotiolo Esperanto Metanotiol Spanish متانتیول Persian
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methanethiol | |||
| Other names
Methyl mercaptan
Mercaptomethane Methiol Thiomethyl alcohol/Thiomethanol Methylthiol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.748 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1064 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH3SH | |||
| Molar mass | 48.11 g·mol−1 | ||
| Appearance | colorless gas[1] | ||
| Odor | Distinctive, like that of rotten cabbage or eggs | ||
| Density | 0.9 g/mL (liquid at 0°C)[1] | ||
| Melting point | −123 °C (−189 °F; 150 K) | ||
| Boiling point | 5.95 °C (42.71 °F; 279.10 K) | ||
| 2% | |||
| Solubility | alcohol, ether | ||
| Vapor pressure | 1.7 atm (20°C)[1] | ||
| Acidity (pKa) | ~10.4 | ||
| Hazards | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H220, H331, H410 | |||
| P210, P261, P271, P273, P304+P340, P311, P321, P377, P381, P391, P403, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −18 °C; 0 °F; 255 K[1] | ||
| 364 °C; 687 °F; 637 K[3] | |||
| Explosive limits | 3.9%-21.8%[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
60.67 mg/kg (mammal)[2] | ||
LC50 (median concentration)
|
3.3 ppm (mouse, 2 hr) 675 ppm (rat, 4 hr)[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
C 10 ppm (20 mg/m3)[1] | ||
REL (Recommended)
|
C 0.5 ppm (1 mg/m3) [15-minute][1] | ||
IDLH (Immediate danger)
|
150 ppm[1] | ||
| Related compounds | |||
Related compounds
|
Ethanethiol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
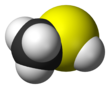
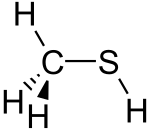
Methanethiol /ˌmɛθ.eɪn.ˈθaɪ.ɒl/ (also known as methyl mercaptan) is an organosulfur compound with the chemical formula CH
3SH. It is a colorless gas with a distinctive putrid smell. It is a natural substance found in the blood, brain and feces of animals (including humans), as well as in plant tissues. It also occurs naturally in certain foods, such as some nuts and cheese. It is one of the chemical compounds responsible for bad breath and the smell of flatus. Methanethiol is the simplest thiol and is sometimes abbreviated as MeSH. It is very flammable.
- ^ a b c d e f g h NIOSH Pocket Guide to Chemical Hazards. "#0425". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Methyl mercaptan". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Sigma Aldrich Methanethiol SDS". Sigma Aldrich. Millipore Sigma. Retrieved Nov 1, 2022.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search