
Back كشف السرطان Arabic غربالگری سرطان Persian Dépistage des cancers en médecine générale French Binciken Ciwon Daji Hausa Screening oncologico Italian がん検診 Japanese 암 검진 Korean Tầm soát ung thư Vietnamese 癌症篩查 Chinese 癌症篩查 ZH-YUE
The examples and perspective in this article deal primarily with the Western world and do not represent a worldwide view of the subject. (March 2024) |
| Cancer screening | |
|---|---|
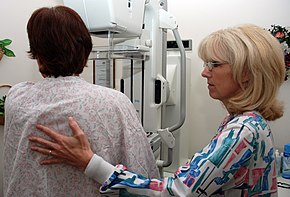 A person preparing for breast cancer screening by mammography | |
| Purpose | detection of cancer prior to onset of symptoms (via several tests/imaging) |
The objective of cancer screening is to detect cancer before symptoms appear, involving various methods such as blood tests, urine tests, DNA tests, and medical imaging.[1][2] The purpose of screening is early cancer detection, to make the cancer easier to treat and extending life expectancy.[3] In 2019, cancer was the second leading cause of death globally; more recent data is pending due to the COVID-19 pandemic.[4]
Universal screening, also known as mass screening or population screening, involves the screening of individuals within certain age and gender groups, aiming to screen the population for particular cancers or cancer risk factors.[5] Selective screening, also known as targeted screening, identifies individuals with a higher risk of developing cancer, including individuals with a family history (genetic risk) of cancer or individuals engaging in high-risk behaviors such as smoking.[5]
The act of cancer screening plays a pivotal role in both preventing cancer and providing early diagnosis, contributing to increased success rates in treatment and ultimately extending life expectancy.[6] Controversy arises when it is not clear if the benefits of the screening outweigh the risks associated with the screening procedure, as well as the subsequent diagnostic tests and cancer treatments.[7] Cancer screening is susceptible to producing both false negative and false positive results, underlining the importance of considering the possible errors in the screening process.[8] Additionally, cancer screening can lead to overtreatment if the screening identifies a tumor that is ultimately benign (non-cancerous).[6]
- ^ "What Is Cancer Screening?". National Cancer Institute. 2010-01-13.
- ^ "Press Announcements - FDA authorizes, with special controls, direct-to-consumer test that reports three mutations in the BRCA breast cancer genes". Food and Drug Administration. 2019-09-10.
- ^ Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, Wender RC (May 2019). "Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening". CA. 69 (3): 184–210. doi:10.3322/caac.21557. PMID 30875085.
- ^ Roser M (December 7, 2021). "Causes of death globally: what do people die from?". Our World in Data.
- ^ a b Bobrowska A, Murton M, Seedat F, Visintin C, Mackie A, Steele R, Marshall J (May 2022). "Targeted screening in the UK: A narrow concept with broad application". The Lancet Regional Health. Europe. 16: 100353. doi:10.1016/j.lanepe.2022.100353. PMC 9038565. PMID 35492962.
- ^ a b Crosby D, Bhatia S, Brindle KM, Coussens LM, Dive C, Emberton M, et al. (March 2022). "Early detection of cancer". Science. 375 (6586): eaay9040. doi:10.1126/science.aay9040. PMID 35298272. S2CID 247521566.
- ^ Shepardson LB, Dean L (August 2020). "Current controversies in breast cancer screening". Seminars in Oncology. 47 (4): 177–181. doi:10.1053/j.seminoncol.2020.05.002. PMID 32513421. S2CID 219550403.
- ^ Miles A, Paschalidi A, Sharma N (September 2023). "The effect of numeric information about the likelihood of receiving a false negative or false positive result on people's attitudes towards colorectal cancer screening using faecal immunochemical testing (FIT)". Patient Education and Counseling. 114: 107857. doi:10.1016/j.pec.2023.107857. PMID 37348310.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search