
Back أكسدة الكحولات Arabic Oxidace alkoholů Czech Oxidación de alcoholes Spanish اکسایش الکل Persian Oxydation d'un alcool French Ossidazione degli alcoli Italian Oxidação de álcool Portuguese 醇的氧化反应 Chinese
Alcohol oxidation is a collection of oxidation reactions in organic chemistry that convert alcohols to aldehydes, ketones, carboxylic acids, and esters where the carbon carries a higher oxidation state. The reaction mainly applies to primary and secondary alcohols. Secondary alcohols form ketones, while primary alcohols form aldehydes or carboxylic acids.[1]
The reaction can occur using a variety of oxidants.
In principle, a simple way to oxidize an alcohols uses an oxygen atom and produce water: In practice, oxygen atoms are unavailable, so the above equation is only conceptual. Instead, most oxidations use oxides-based reagents, such as metal oxo complexes, sulfoxides, and iodine oxides.
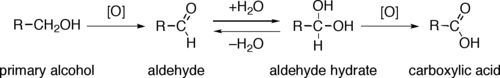
Through a variety of mechanisms, the removal of a hydride equivalent converts a primary or secondary alcohol to an aldehyde or ketone, respectively. The oxidation of primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde, which is transformed via an aldehyde hydrate (gem-diol, R-CH(OH)2) by reaction with water. Thus, the oxidation of a primary alcohol at the aldehyde level without further oxidation to the carboxylic acid is possible by performing the reaction in absence of water, so that no aldehyde hydrate can be formed.
- ^ Burton, George et al. (2000). Salters Advanced Chemistry: Chemical (2nd ed.). Heinemann. ISBN 0-435-63120-9
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search